当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unveiling solvation structure and desolvation dynamics of hybrid electrolytes for ultralong cyclability and facile kinetics of Zn–Al alloy anodes
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2022-09-15 , DOI: 10.1039/d2ee02453e Qingyun Dou 1 , Nan Yao 2 , Wei Kong Pang 3 , Yeonju Park 4 , Peixun Xiong 1 , Xiaotong Han 1 , Harpalsinh H. Rana 1 , Xiang Chen 2 , Zhong-Heng Fu 2 , Lars Thomsen 5 , Bruce Cowie 5 , Yingbo Kang 1 , Qin Liu 1 , Dong Hyun Min 1 , Young Mee Jung 4, 6 , Zaiping Guo 7 , Qiang Zhang 2 , Ho Seok Park 1, 8, 9
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2022-09-15 , DOI: 10.1039/d2ee02453e Qingyun Dou 1 , Nan Yao 2 , Wei Kong Pang 3 , Yeonju Park 4 , Peixun Xiong 1 , Xiaotong Han 1 , Harpalsinh H. Rana 1 , Xiang Chen 2 , Zhong-Heng Fu 2 , Lars Thomsen 5 , Bruce Cowie 5 , Yingbo Kang 1 , Qin Liu 1 , Dong Hyun Min 1 , Young Mee Jung 4, 6 , Zaiping Guo 7 , Qiang Zhang 2 , Ho Seok Park 1, 8, 9
Affiliation
|
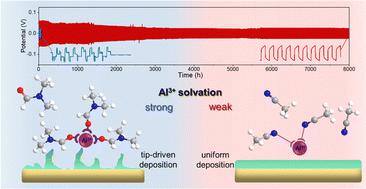
Despite the high theoretical capacity and natural abundance of Al metal anodes, the reversible and fast multivalent storage of Al3+ ions remains challenging because their large charge density leads to strong electrostatic interactions with other components and sluggish kinetics. Herein, we report the record-high plating/stripping time (>8000 h) and high rate capability of Zn–Al alloy anodes in Al3+-containing hybrid electrolytes. The more reversible Al deposition on Zn in nitrile-based hybrid electrolyte than carbonate- and amide-based hybrid and aqueous electrolytes is attributed to weak Al3+–solvent interactions and fast Al3+ transfer kinetics. In particular, these electrochemical behaviors of nitrile-based electrolyte originate from a unique solvation structure, the interrelation among H2O, organic solvents, and Al3+, and the conformational change of bound/free solvents upon desolvation, as elaborated via theoretical simulations, two-dimensional infrared correlation spectroscopy, and other characterizations. The superiority of this hybrid electrolyte was confirmed by achieving a high specific capacity (183 mA h g−1 and 1.08 mA h cm−2) and long cycling of >5000 cycles of full cells integrating Zn–Al alloy anodes (25 μm) with vanadium dioxide/carbon nanotubes (8 mg cm−2) and activated carbon (10 mg cm−2) cathodes, respectively, which considerably exceed those of Al-based full cells.
中文翻译:

揭示混合电解质的溶剂化结构和去溶剂化动力学,以实现 Zn-Al 合金负极的超长循环性和简便动力学
尽管 Al 金属负极具有高理论容量和天然丰度,但 Al 3+离子的可逆和快速多价存储仍然具有挑战性,因为它们的大电荷密度导致与其他组分的强静电相互作用和缓慢的动力学。在此,我们报告了 Zn-Al 合金负极在含 Al 3+的混合电解质中的创纪录的电镀/剥离时间(>8000 小时)和高倍率性能。与碳酸盐和酰胺基混合电解质和水性电解质相比,腈基混合电解质中锌上更可逆的 Al 沉积归因于弱 Al 3+ -溶剂相互作用和快速 Al 3+转移动力学。特别是,腈基电解质的这些电化学行为源于独特的溶剂化结构、H 2 O、有机溶剂和 Al 3+之间的相互关系,以及结合/游离溶剂在去溶剂化时的构象变化,正如通过理论模拟所阐述的那样,二维红外相关光谱和其他表征。这种混合电解质的优越性通过实现高比容量(183 mA hg -1和 1.08 mA h cm -2)和集成 Zn-Al 合金阳极(25 μm)与钒的全电池的 >5000 次循环的长循环得到证实二氧化碳/碳纳米管 (8 mg cm -2 ) 和活性炭 (10 mg cm-2 ) 阴极,分别显着超过基于铝的全电池。
更新日期:2022-09-15
中文翻译:

揭示混合电解质的溶剂化结构和去溶剂化动力学,以实现 Zn-Al 合金负极的超长循环性和简便动力学
尽管 Al 金属负极具有高理论容量和天然丰度,但 Al 3+离子的可逆和快速多价存储仍然具有挑战性,因为它们的大电荷密度导致与其他组分的强静电相互作用和缓慢的动力学。在此,我们报告了 Zn-Al 合金负极在含 Al 3+的混合电解质中的创纪录的电镀/剥离时间(>8000 小时)和高倍率性能。与碳酸盐和酰胺基混合电解质和水性电解质相比,腈基混合电解质中锌上更可逆的 Al 沉积归因于弱 Al 3+ -溶剂相互作用和快速 Al 3+转移动力学。特别是,腈基电解质的这些电化学行为源于独特的溶剂化结构、H 2 O、有机溶剂和 Al 3+之间的相互关系,以及结合/游离溶剂在去溶剂化时的构象变化,正如通过理论模拟所阐述的那样,二维红外相关光谱和其他表征。这种混合电解质的优越性通过实现高比容量(183 mA hg -1和 1.08 mA h cm -2)和集成 Zn-Al 合金阳极(25 μm)与钒的全电池的 >5000 次循环的长循环得到证实二氧化碳/碳纳米管 (8 mg cm -2 ) 和活性炭 (10 mg cm-2 ) 阴极,分别显着超过基于铝的全电池。



























 京公网安备 11010802027423号
京公网安备 11010802027423号