当前位置:
X-MOL 学术
›
J. Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Self-assembly of a peptide sequence, EKKE, composed of exclusively charged amino acids: Role of charge in morphology and lead binding
Journal of Peptide Science ( IF 2.1 ) Pub Date : 2022-09-13 , DOI: 10.1002/psc.3451 Aishwarya Natarajan 1 , Krishnan Rangan 2 , Ramakrishna Vadrevu 1
Journal of Peptide Science ( IF 2.1 ) Pub Date : 2022-09-13 , DOI: 10.1002/psc.3451 Aishwarya Natarajan 1 , Krishnan Rangan 2 , Ramakrishna Vadrevu 1
Affiliation
|
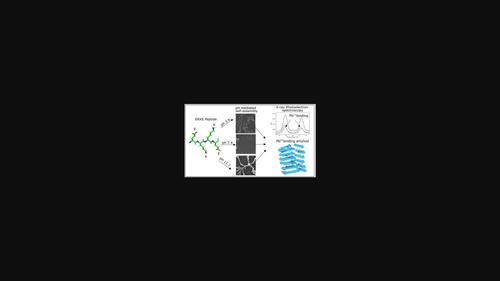
The self-assembly of peptides is influenced by their amino acid sequence and other factors including pH, charge, temperature, and solvent. Herein, we explore whether a four-residue sequence, EKKE, consisting of exclusively charged amino acids shows the propensity to form self-assembled ordered nanostructures and whether the overall charge plays any role in morphological and functional properties. From a combination of experimental data provided by Thioflavin T fluorescence, Congo red absorbance, circular dichroism spectroscopy, dynamic light scattering, field emission-scanning electron microscopy, atomic force microscopy, and confocal microscopy, it is clear that the all-polar peptide and charged EKKE sequence shows a pH-dependent tendency to form amyloid-like structures, and the self-assembled entities under acidic, basic and neutral conditions exhibit morphological variation. Additionally, the ability of the self-assembled amyloid nanostructures to bind to the toxic metal, lead (Pb2+), was demonstrated from the analysis of the ultraviolet absorbance and X-ray photoelectron spectroscopy data. The modulation at the sequence level for the amyloid-forming EKKE scaffold can further extend its potential role not only in the remediation of other toxic metals but also towards biomedical applications.
中文翻译:

由完全带电的氨基酸组成的肽序列 EKKE 的自组装:电荷在形态学和铅结合中的作用
肽的自组装受其氨基酸序列和其他因素(包括 pH、电荷、温度和溶剂)的影响。在此,我们探讨了由完全带电的氨基酸组成的四残基序列 EKKE 是否显示出形成自组装有序纳米结构的倾向,以及总电荷是否在形态和功能特性中发挥任何作用。结合硫黄素 T 荧光、刚果红吸光度、圆二色光谱、动态光散射、场发射扫描电子显微镜、原子力显微镜和共聚焦显微镜提供的实验数据,很明显,全极性肽和带电EKKE 序列显示出形成淀粉样蛋白样结构的 pH 依赖性趋势,并且在酸性下自组装实体,基本和中性条件表现出形态变异。此外,自组装淀粉样蛋白纳米结构与有毒金属铅(Pb2+ ),通过紫外吸光度和X射线光电子能谱数据的分析得到证实。淀粉样蛋白形成 EKKE 支架在序列水平上的调节可以进一步扩展其潜在作用,不仅在其他有毒金属的修复中,而且在生物医学应用中。
更新日期:2022-09-13
中文翻译:

由完全带电的氨基酸组成的肽序列 EKKE 的自组装:电荷在形态学和铅结合中的作用
肽的自组装受其氨基酸序列和其他因素(包括 pH、电荷、温度和溶剂)的影响。在此,我们探讨了由完全带电的氨基酸组成的四残基序列 EKKE 是否显示出形成自组装有序纳米结构的倾向,以及总电荷是否在形态和功能特性中发挥任何作用。结合硫黄素 T 荧光、刚果红吸光度、圆二色光谱、动态光散射、场发射扫描电子显微镜、原子力显微镜和共聚焦显微镜提供的实验数据,很明显,全极性肽和带电EKKE 序列显示出形成淀粉样蛋白样结构的 pH 依赖性趋势,并且在酸性下自组装实体,基本和中性条件表现出形态变异。此外,自组装淀粉样蛋白纳米结构与有毒金属铅(Pb2+ ),通过紫外吸光度和X射线光电子能谱数据的分析得到证实。淀粉样蛋白形成 EKKE 支架在序列水平上的调节可以进一步扩展其潜在作用,不仅在其他有毒金属的修复中,而且在生物医学应用中。


























 京公网安备 11010802027423号
京公网安备 11010802027423号