当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrocatalytic selectivity for nitrogen reduction vs. hydrogen evolution: a comparison of vanadium and cobalt oxynitrides at different pH values
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2022-09-13 , DOI: 10.1039/d2ta05180j Precious Chukwunenye 1 , Ashwin Ganesan 1 , Mojgan Gharaee 1 , Kabirat Balogun 1 , Fatima Anwar 1 , Qasim Adesope 1 , Thomas R. Cundari 1 , Francis D'Souza 1 , Jeffry A. Kelber 1
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2022-09-13 , DOI: 10.1039/d2ta05180j Precious Chukwunenye 1 , Ashwin Ganesan 1 , Mojgan Gharaee 1 , Kabirat Balogun 1 , Fatima Anwar 1 , Qasim Adesope 1 , Thomas R. Cundari 1 , Francis D'Souza 1 , Jeffry A. Kelber 1
Affiliation
|
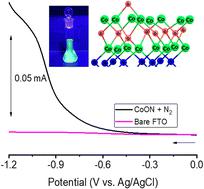
The electrocatalytic nitrogen reduction reaction (NRR) is of significant interest as an environmentally friendly method for NH3 production for agricultural and clean energy applications. Selectivity of NRR vis-à-vis the hydrogen evolution reaction (HER), however, is thought to adversely impact many potential catalysts, including Earth-abundant transition metal oxynitrides. Relative HER/NRR selectivities are therefore directly compared for two transition metal oxynitrides with different metal oxophilicities—Co and V. Electrocatalytic current–potential measurements, operando fluorescence, absorption, and GC measurements of H2 and NH3 production, ex situ X-ray photoelectron spectroscopy (XPS), and density functional theory (DFT) calculations are combined to directly compare NRR and HER activities under identical reaction conditions. Results show that cobalt oxynitrides – with Co primarily in the Co(II) oxidation state – are NRR active at pH 10, with electrochemical reduction of both lattice nitrogen and dissolved N2, the latter occurring without N incorporation into the lattice. Removal of lattice N then yields Co(II) oxide, which is still NRR active. These results are complemented by calculations showing that N2 binding at Co(II) sites is energetically favored over binding at Co(III) sites. GC analysis demonstrates that H2 production occurs in concert with ammonia production but at a far greater rate. In contrast, vanadium oxynitride films are HER inactive under the same (pH 10) conditions, as well as at pH 7, but are NRR active at pH 7. DFT calculations indicate that a major difference in the two materials is hindered O–H dissociation of H2O adsorbed at O-ligated Co vs. V cation centers. The combined studies indicate significant variation in HER vs. NRR selectivity as a function of employed transition metal oxynitrides, as well as different HER mechanisms in V and Co oxynitrides.
中文翻译:

氮还原与析氢的电催化选择性:不同 pH 值下钒和钴氮氧化物的比较
电催化氮还原反应 (NRR) 作为用于农业和清洁能源应用的 NH 3生产的环保方法具有重要意义。然而,NRR 相对于析氢反应 (HER) 的选择性被认为会对许多潜在的催化剂产生不利影响,包括地球上丰富的过渡金属氧氮化物。因此,直接比较了两种具有不同金属亲氧性的过渡金属氧氮化物(Co 和 V)的相对 HER/NRR 选择性。H 2和 NH 3非原位生成的电催化电流-电位测量、操作荧光、吸收和 GC 测量X 射线光电子能谱 (XPS) 和密度泛函理论 (DFT) 计算相结合,可直接比较相同反应条件下的 NRR 和 HER 活性。结果表明,钴的氧氮化物——Co 主要处于 Co( II ) 氧化态——在 pH 10 时具有 NRR 活性,晶格氮和溶解的 N 2均发生电化学还原,后者在没有 N 掺入晶格的情况下发生。去除晶格 N 然后产生 Co( II ) 氧化物,它仍然是 NRR 活性的。计算结果补充了这些结果,显示在 Co(II) 位点的 N 2结合比在 Co( III ) 位点的结合更受青睐。GC 分析表明 H2的产生与氨的产生同时发生,但速度要快得多。相比之下,氧氮化钒薄膜在相同(pH 10)条件下以及在 pH 7 下均不具有 HER 活性,但在 pH 7 下具有 NRR 活性。DFT 计算表明两种材料的主要区别在于受阻的 O-H 解离H 2 O 吸附在 O 配位的 Co对V 阳离子中心。综合研究表明 HER与NRR 选择性的显着变化是所采用的过渡金属氮氧化物的函数,以及 V 和 Co 氮氧化物中的不同 HER 机制。
更新日期:2022-09-13
中文翻译:

氮还原与析氢的电催化选择性:不同 pH 值下钒和钴氮氧化物的比较
电催化氮还原反应 (NRR) 作为用于农业和清洁能源应用的 NH 3生产的环保方法具有重要意义。然而,NRR 相对于析氢反应 (HER) 的选择性被认为会对许多潜在的催化剂产生不利影响,包括地球上丰富的过渡金属氧氮化物。因此,直接比较了两种具有不同金属亲氧性的过渡金属氧氮化物(Co 和 V)的相对 HER/NRR 选择性。H 2和 NH 3非原位生成的电催化电流-电位测量、操作荧光、吸收和 GC 测量X 射线光电子能谱 (XPS) 和密度泛函理论 (DFT) 计算相结合,可直接比较相同反应条件下的 NRR 和 HER 活性。结果表明,钴的氧氮化物——Co 主要处于 Co( II ) 氧化态——在 pH 10 时具有 NRR 活性,晶格氮和溶解的 N 2均发生电化学还原,后者在没有 N 掺入晶格的情况下发生。去除晶格 N 然后产生 Co( II ) 氧化物,它仍然是 NRR 活性的。计算结果补充了这些结果,显示在 Co(II) 位点的 N 2结合比在 Co( III ) 位点的结合更受青睐。GC 分析表明 H2的产生与氨的产生同时发生,但速度要快得多。相比之下,氧氮化钒薄膜在相同(pH 10)条件下以及在 pH 7 下均不具有 HER 活性,但在 pH 7 下具有 NRR 活性。DFT 计算表明两种材料的主要区别在于受阻的 O-H 解离H 2 O 吸附在 O 配位的 Co对V 阳离子中心。综合研究表明 HER与NRR 选择性的显着变化是所采用的过渡金属氮氧化物的函数,以及 V 和 Co 氮氧化物中的不同 HER 机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号