当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Vapor–Liquid Equilibrium (P–T–x–y) Measurements and Modeling of propan-1-ol/propan-2-ol + oct-1-ene in the Range of T = (313.2–353.2) K
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2022-09-13 , DOI: 10.1021/acs.jced.2c00466 Kuveneshan Moodley 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2022-09-13 , DOI: 10.1021/acs.jced.2c00466 Kuveneshan Moodley 1
Affiliation
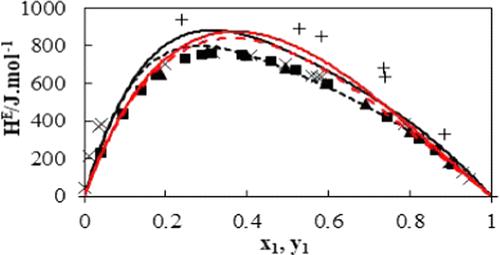
|
In this work, the isothermal vapor–liquid equilibrium (VLE) behavior was measured for systems consisting of propan-1-ol/propan-2-ol with oct-1-ene using a dynamic glass apparatus. The temperatures measured included (T = 313.2, 333.2, 353.2) K, which resulted in sub-atmospheric total pressures. The systems exhibit highly nonideal azeotropic behavior and large relative volatilities. The VLE data were correlated using the combined (γ-Φ) method, with the nonrandom two-liquid (NRTL) or universal quasi-chemical (UNIQUAC) activity coefficient models employed to account for the nonideality of the liquid phase, and the virial equation of state to describe the nonideality of the vapor phase. The nonrandom two-liquid model with the virial equation of state performed better than the UNIQUAC model with the virial equation of state in fitting the experimental data. The data for all systems were found to be thermodynamically consistent according to the area, point, and infinite dilution tests.
更新日期:2022-09-13



























 京公网安备 11010802027423号
京公网安备 11010802027423号