当前位置:
X-MOL 学术
›
Cancer Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Targeting the PTP1B-Bcr-Abl1 interaction for the degradation of T315I mutant Bcr-Abl1 in chronic myeloid leukemia
Cancer Science ( IF 5.7 ) Pub Date : 2022-09-10 , DOI: 10.1111/cas.15580 Ahmed Elgehama 1 , Yixuan Wang 1 , Ying Yu 1 , Lin Zhou 1 , Zhixiu Chen 1 , Liwei Wang 1 , Lijun Sun 2 , Jian Gao 1 , Biao Yu 3 , Yan Shen 1 , Qiang Xu 1
Cancer Science ( IF 5.7 ) Pub Date : 2022-09-10 , DOI: 10.1111/cas.15580 Ahmed Elgehama 1 , Yixuan Wang 1 , Ying Yu 1 , Lin Zhou 1 , Zhixiu Chen 1 , Liwei Wang 1 , Lijun Sun 2 , Jian Gao 1 , Biao Yu 3 , Yan Shen 1 , Qiang Xu 1
Affiliation
|
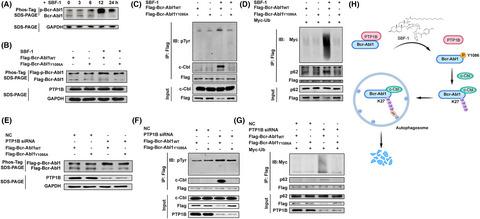
Small-molecule-induced degradation of mutant Bcr-Abl1 provides a potential approach to overcome Bcr-Abl1 tyrosine kinase inhibitor (TKI)-resistant chronic myeloid leukemia (CML). Our previous study reported that a synthetic steroidal glycoside SBF-1 showed remarkable anti-CML activity by inducing the degradation of native Bcr-Abl1 protein. Here, we observed the comparable growth inhibition for SBF-1 in CML cells harboring T315I mutant Bcr-Abl1 in vitro and in vivo. SBF-1 triggered its degradation through disrupting the interaction between protein-tyrosine phosphatase 1B (PTP1B) and Bcr-Abl1. Using SBF-1 as a tool, we found that Tyr46 in the PTP1B catalytic domain and Tyr852 in the Bcr-Abl1 pleckstrin-homology (PH) domain are critical for their interaction. Moreover, the phosphorylation of Tyr1086 within the Bcr-Abl1 SH2 domain recruited the E3 ubiquitin ligase c-Cbl to catalyze K27-linked ubiquitin chains, which serve as a recognition signal for p62-dependent autophagic degradation. PTP1B dephosphorylated Bcr-Abl1 at Tyr1086 and prevented the recruitment of c-Cbl, leading to the stability of Bcr-Abl1. This study unravels the action mechanism of PTP1B in stabilizing Bcr-Abl1 protein and indicates that the PTP1B-Bcr-Abl1 interaction might be one of druggable targets for TKI-resistant CML with point mutations.
中文翻译:

针对慢性粒细胞白血病中 T315I 突变体 Bcr-Abl1 降解的 PTP1B-Bcr-Abl1 相互作用
小分子诱导的突变体 Bcr-Abl1 降解提供了一种潜在的方法来克服 Bcr-Abl1 酪氨酸激酶抑制剂 (TKI) 耐药的慢性粒细胞白血病 (CML)。我们之前的研究报道,合成的甾体糖苷 SBF-1 通过诱导天然 Bcr-Abl1 蛋白的降解显示出显着的抗 CML 活性。在这里,我们在体外和体内观察到 SBF-1 在携带 T315I 突变体 Bcr-Abl1 的 CML 细胞中的类似生长抑制。SBF-1 通过破坏蛋白酪氨酸磷酸酶 1B (PTP1B) 和 Bcr-Abl1 之间的相互作用触发其降解。使用 SBF-1 作为工具,我们发现 PTP1B 催化结构域中的 Tyr46 和 Bcr-Abl1 pleckstrin-同源 (PH) 结构域中的 Tyr852 对于它们的相互作用至关重要。而且,Bcr-Abl1 SH2 结构域内 Tyr1086 的磷酸化募集 E3 泛素连接酶 c-Cbl 催化 K27 连接的泛素链,作为 p62 依赖性自噬降解的识别信号。PTP1B 在 Tyr1086 位点使 Bcr-Abl1 去磷酸化并阻止 c-Cbl 的募集,从而导致 Bcr-Abl1 的稳定性。本研究揭示了 PTP1B 在稳定 Bcr-Abl1 蛋白中的作用机制,并表明 PTP1B-Bcr-Abl1 相互作用可能是具有点突变的 TKI 耐药 CML 的药物靶点之一。
更新日期:2022-09-10
中文翻译:

针对慢性粒细胞白血病中 T315I 突变体 Bcr-Abl1 降解的 PTP1B-Bcr-Abl1 相互作用
小分子诱导的突变体 Bcr-Abl1 降解提供了一种潜在的方法来克服 Bcr-Abl1 酪氨酸激酶抑制剂 (TKI) 耐药的慢性粒细胞白血病 (CML)。我们之前的研究报道,合成的甾体糖苷 SBF-1 通过诱导天然 Bcr-Abl1 蛋白的降解显示出显着的抗 CML 活性。在这里,我们在体外和体内观察到 SBF-1 在携带 T315I 突变体 Bcr-Abl1 的 CML 细胞中的类似生长抑制。SBF-1 通过破坏蛋白酪氨酸磷酸酶 1B (PTP1B) 和 Bcr-Abl1 之间的相互作用触发其降解。使用 SBF-1 作为工具,我们发现 PTP1B 催化结构域中的 Tyr46 和 Bcr-Abl1 pleckstrin-同源 (PH) 结构域中的 Tyr852 对于它们的相互作用至关重要。而且,Bcr-Abl1 SH2 结构域内 Tyr1086 的磷酸化募集 E3 泛素连接酶 c-Cbl 催化 K27 连接的泛素链,作为 p62 依赖性自噬降解的识别信号。PTP1B 在 Tyr1086 位点使 Bcr-Abl1 去磷酸化并阻止 c-Cbl 的募集,从而导致 Bcr-Abl1 的稳定性。本研究揭示了 PTP1B 在稳定 Bcr-Abl1 蛋白中的作用机制,并表明 PTP1B-Bcr-Abl1 相互作用可能是具有点突变的 TKI 耐药 CML 的药物靶点之一。



























 京公网安备 11010802027423号
京公网安备 11010802027423号