Journal of Hepatology ( IF 25.7 ) Pub Date : 2022-09-07 , DOI: 10.1016/j.jhep.2022.08.035 Tiong Y Lim 1 , Elena Perpiñán 1 , Maria-Carlota Londoño 2 , Rosa Miquel 3 , Paula Ruiz 1 , Ada S Kurt 1 , Elisavet Kodela 1 , Amy R Cross 4 , Claudia Berlin 4 , Joanna Hester 4 , Fadi Issa 4 , Abdel Douiri 5 , Felix H Volmer 6 , Richard Taubert 6 , Evangelia Williams 7 , Anthony J Demetris 8 , Andrew Lesniak 8 , Gilbert Bensimon 9 , Juan José Lozano 10 , Marc Martinez-Llordella 1 , Tim Tree 7 , Alberto Sánchez-Fueyo 1
|
Background & Aims
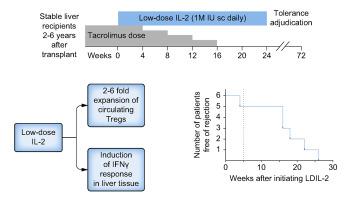
CD4+CD25+Foxp3+ regulatory T cells (Tregs) are essential to maintain immunological tolerance and have been shown to promote liver allograft tolerance in both rodents and humans. Low-dose IL-2 (LDIL-2) can expand human endogenous circulating Tregs in vivo, but its role in suppressing antigen-specific responses and promoting Treg trafficking to the sites of inflammation is unknown. Likewise, whether LDIL-2 facilitates the induction of allograft tolerance has not been investigated in humans.
Methods
We conducted a clinical trial in stable liver transplant recipients 2–6 years post-transplant to determine the capacity of LDIL-2 to suppress allospecific immune responses and allow for the complete discontinuation of maintenance immunosuppression (ClinicalTrials.gov NCT02949492). One month after LDIL-2 was initiated, those exhibiting at least a 2-fold increase in circulating Tregs gradually discontinued immunosuppression over a 4-month period while continuing LDIL-2 for a total treatment duration of 6 months.
Results
All participants achieved a marked and sustained increase in circulating Tregs. However, this was not associated with the preferential expansion of donor-reactive Tregs and did not promote the accumulation of intrahepatic Tregs. Furthermore, LDIL-2 induced a marked IFNγ-orchestrated transcriptional response in the liver even before immunosuppression weaning was initiated. The trial was terminated after the first 6 participants failed to reach the primary endpoint owing to rejection requiring reinstitution of immunosuppression.
Conclusions
The expansion of circulating Tregs in response to LDIL-2 is not sufficient to control alloimmunity and to promote liver allograft tolerance, due, at least in part, to off-target effects that increase liver immunogenicity. Our trial provides unique insight into the mechanisms of action of immunomodulatory therapies such as LDIL-2 and their limitations in promoting alloantigen-specific effects and immunological tolerance.
Clinical Trials Registration
The study is registered at ClinicalTrials.gov (NCT02949492).
Impact and implications
The administration of low-dose IL-2 is an effective way of increasing the number of circulating regulatory T cells (Tregs), an immunosuppressive lymphocyte subset that is key for the establishment of immunological tolerance, but its use to promote allograft tolerance in the setting of clinical liver transplantation had not been explored before. In liver transplant recipients on tacrolimus monotherapy, low-dose IL-2 effectively expanded circulating Tregs but did not increase the number of Tregs with donor specificity, nor did it promote their trafficking to the transplanted liver. Low-dose IL-2 did not facilitate the discontinuation of tacrolimus and elicited, as an off-target effect, an IFNγ-orchestrated inflammatory response in the liver that resembled T cell-mediated rejection. These results, supporting an unexpected role for IL-2 in regulating the immunogenicity of the liver, highlight the need to carefully evaluate systemic immunoregulatory strategies with investigations that are not restricted to the blood compartment and involve target tissues such as the liver.
中文翻译:

低剂量白细胞介素 2 选择性地扩增循环调节性 T 细胞,但未能促进人类肝脏同种异体移植耐受性
背景与目标
CD4 + CD25 + Foxp3 +调节性 T 细胞 (Treg) 对维持免疫耐受至关重要,并已被证明可促进啮齿动物和人类的肝脏同种异体移植耐受性。低剂量 IL-2 (LDIL-2) 可以在体内扩增人内源性循环 Tregs ,但其在抑制抗原特异性反应和促进 Treg 运输至炎症部位方面的作用尚不清楚。同样,尚未在人类中研究 LDIL-2 是否促进同种异体移植物耐受性的诱导。
方法
我们在移植后 2-6 年对稳定的肝移植受者进行了一项临床试验,以确定 LDIL-2 抑制同种异体特异性免疫反应的能力,并允许完全停止维持免疫抑制 (ClinicalTrials.gov NCT02949492)。开始使用 LDIL-2 一个月后,循环 Tregs 至少增加 2 倍的患者在 4 个月的时间内逐渐停止免疫抑制,同时继续使用 LDIL-2 进行 6 个月的总治疗。
结果
所有参与者都实现了循环 Treg 的显着和持续增加。然而,这与供体反应性 Treg 的优先扩增无关,也没有促进肝内 Treg 的积累。此外,甚至在免疫抑制断奶开始之前,LDIL-2 就在肝脏中诱导了显着的 IFNγ 协调的转录反应。在前 6 名参与者因排斥需要重新进行免疫抑制而未能达到主要终点后,该试验终止。
结论
响应 LDIL-2 的循环 Treg 的扩增不足以控制同种免疫和促进肝脏同种异体移植耐受性,至少部分是由于增加肝脏免疫原性的脱靶效应。我们的试验对 LDIL-2 等免疫调节疗法的作用机制及其在促进同种异体抗原特异性作用和免疫耐受方面的局限性提供了独特的见解。
临床试验注册
该研究已在 ClinicalTrials.gov (NCT02949492) 上注册。
影响和启示
施用低剂量 IL-2 是增加循环调节性 T 细胞 (Treg) 数量的有效方法,Tregs 是一种免疫抑制性淋巴细胞亚群,是建立免疫耐受的关键,但它在环境中用于促进同种异体移植耐受以前没有探索过临床肝移植。在接受他克莫司单一疗法的肝移植受者中,低剂量 IL-2 有效地扩大了循环 Tregs,但没有增加具有供体特异性的 Tregs 数量,也没有促进它们向移植肝脏的运输。低剂量 IL-2 不会促进他克莫司的停药,并且作为脱靶效应在肝脏中引发类似于 T 细胞介导的排斥反应的 IFNγ 协调的炎症反应。这些结果,

























 京公网安备 11010802027423号
京公网安备 11010802027423号