Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2022-09-08 , DOI: 10.1016/j.bmcl.2022.128981 Young-Hwan Jung 1 , Qasim Shah 2 , Sarah A Lewicki 1 , Asmita Pramanik 1 , Varun Gopinatth 1 , Julie Pelletier 3 , Jean Sévigny 3 , Jamshed Iqbal 4 , Kenneth A Jacobson 1
|
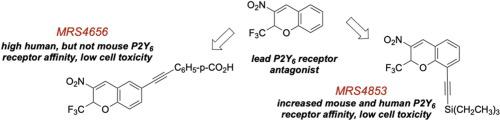
P2Y6 receptor (P2Y6R) antagonists represent potential drugs for treating cancer, pain, neurodegeneration, asthma, diabetes, colitis and other disorders. However, there are few chemical classes of known competitive antagonists. We recently explored the structure activity relationship (SAR) of 2H-chromene derivatives as P2Y6R antagonists of moderate affinity. New analogues in this series modified at five positions were synthesized and shown to antagonize Ca2+ transients induced by the native agonist UDP in human (h) P2Y6R-expressing (but not turkey P2Y1R-, hP2Y2R- or hP2Y4R-expressing) astrocytoma cells. Alternatives to the reported 2-(trifluoromethyl)- and 3-nitro- substitutions of this scaffold were not identified. However, 6‑fluoro 11 and 6‑chloro 12 analogues displayed enhanced potency compared to other halogens, although still in the 1 – 2 µM range. Similar halogen substitution at 5, 7 or 8 positions reduced affinity. 5- or 8‑Triethylsilylethynyl extension maintained hP2Y6R affinity, with IC50 0.46 µM for 26 (MRS4853). The 6,8‑difluoro analogue 27 (IC50 2.99 µM) lacked off-target activities among 45 sites examined, unlike earlier analogues that bound to biogenic amine receptors. 11 displayed only one weak off-target activity (σ2). Mouse P2Y6R IC50s of 5, 25, 26 and 27 were 4.94, 17.6, 6.15 and 17.8 µM, respectively, but most other analogues had reduced affinity (>20 µM) compared to the hP2Y6R. These analogues are suitable for evaluation in in vivo inflammation and cancer models, which will be performed in the future studies.
中文翻译:

多取代 2H-色烯衍生物作为 P2Y6 受体拮抗剂的合成和药理学表征
P2Y 6受体(P2Y 6 R)拮抗剂代表了用于治疗癌症、疼痛、神经变性、哮喘、糖尿病、结肠炎和其他疾病的潜在药物。然而,已知的竞争性拮抗剂的化学类别很少。我们最近探索了 2 H-色烯衍生物作为中等亲和力的 P2Y 6 R 拮抗剂的结构活性关系 (SAR)。合成了该系列中在五个位置进行修饰的新类似物,并显示拮抗由天然激动剂 UDP 在人类 (h) P2Y 6 R 表达(但不是火鸡 P2Y 1 R-、hP2Y 2 R-或 hP2Y 4R-表达)星形细胞瘤细胞。尚未确定报告的该支架的 2-(三氟甲基)和 3-硝基取代的替代品。然而,与其他卤素相比,6-氟11和 6-氯12类似物显示出增强的效力,尽管仍在 1 – 2 µM 范围内。5、7 或 8 位的类似卤素取代降低了亲和力。5-或 8-三乙基甲硅烷基乙炔基延伸保持 hP2Y 6 R 亲和力,26 (MRS4853)的 IC 50为 0.46 µM 。与早期的与生物胺受体结合的类似物不同,6,8-二氟类似物27 (IC 50 2.99 µM) 在所检查的 45 个位点中缺乏脱靶活性。11仅显示一种微弱的脱靶活动 (σ 2 )。5、25、26和27的小鼠 P2Y 6 R IC 50 s分别为4.94、17.6、6.15和 17.8 µM,但与 hP2Y 6 R 相比,大多数其他类似物的亲和力降低 (>20 µM) 。这些类似物适用用于评估体内炎症和癌症模型,这将在未来的研究中进行。



























 京公网安备 11010802027423号
京公网安备 11010802027423号