当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Thermophysical Studies on Molecular Interactions of Semicarbazide Hydrochloride/Domiphen Bromide in Aqueous Deep Eutectic Solvent Media at Various Temperatures
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2022-09-01 , DOI: 10.1021/acs.jced.2c00413 Qammer Majid 1 , Richu 1 , Himani Singh 1 , Akshita Bandral 1 , Ashwani Kumar 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2022-09-01 , DOI: 10.1021/acs.jced.2c00413 Qammer Majid 1 , Richu 1 , Himani Singh 1 , Akshita Bandral 1 , Ashwani Kumar 1
Affiliation
|
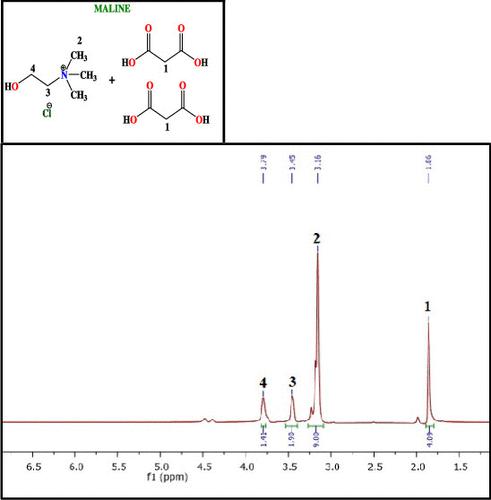
A comprehensive examination of the thermophysical properties of semicarbazide hydrochloride and domiphen bromide in water and aqueous maline solutions (maline, a deep eutectic solvent (DES) made by mixing choline chloride and malonic acid in 1:2 mol ratio) has been done by a combination of ultrasonic, volumetric, viscometric, and thermodynamic methods at the temperature range from 293.15 to 318.15 K. Exploration of molecular interactions through the procured thermophysical properties is very important because it provides an insight into the forces that are operating in the liquid mixtures. In the present study, thermophysical parameters such as apparent molar volume (Vϕ), apparent molar isentropic compressibility (Kϕ,s), viscosity coefficients, Hepler’s constant, etc. are studied for the above-mentioned drugs in water and aqueous maline solutions. The results revealed the dominance of solute–solvent interactions in the studied systems, which were found to escalate with elevation in temperature and with the concentration of maline. The results are discussed in terms of hydrophilic–hydrophilic and hydrophilic–hydrophobic interactions in these systems along with the structure breaking/making ability of the drugs. The co-sphere overlap model has been used to interpret the positive transfer values.
中文翻译:

不同温度下水性深共晶溶剂介质中盐酸氨基脲/多米芬分子间相互作用的热物理研究
对氨基脲盐酸盐和多米芬溴化物在水和马来酸水溶液(马来酸,一种由氯化胆碱和丙二酸以 1:2 摩尔比混合制成的深共熔溶剂 (DES))中的热物理性质进行了综合检查在 293.15 至 318.15 K 的温度范围内进行超声波、体积、粘度和热力学方法的研究。通过获得的热物理性质探索分子相互作用非常重要,因为它提供了对液体混合物中作用力的洞察力。在本研究中,表观摩尔体积 ( V φ )、表观摩尔等熵压缩率 ( K φ,s)、粘度系数、赫普勒常数等研究了上述药物在水和马林水溶液中的情况。结果揭示了所研究系统中溶质-溶剂相互作用的主导地位,发现其随着温度升高和马林浓度而升高。根据这些系统中的亲水-亲水和亲水-疏水相互作用以及药物的结构破坏/制造能力来讨论结果。共球重叠模型已用于解释正转移值。
更新日期:2022-09-01
中文翻译:

不同温度下水性深共晶溶剂介质中盐酸氨基脲/多米芬分子间相互作用的热物理研究
对氨基脲盐酸盐和多米芬溴化物在水和马来酸水溶液(马来酸,一种由氯化胆碱和丙二酸以 1:2 摩尔比混合制成的深共熔溶剂 (DES))中的热物理性质进行了综合检查在 293.15 至 318.15 K 的温度范围内进行超声波、体积、粘度和热力学方法的研究。通过获得的热物理性质探索分子相互作用非常重要,因为它提供了对液体混合物中作用力的洞察力。在本研究中,表观摩尔体积 ( V φ )、表观摩尔等熵压缩率 ( K φ,s)、粘度系数、赫普勒常数等研究了上述药物在水和马林水溶液中的情况。结果揭示了所研究系统中溶质-溶剂相互作用的主导地位,发现其随着温度升高和马林浓度而升高。根据这些系统中的亲水-亲水和亲水-疏水相互作用以及药物的结构破坏/制造能力来讨论结果。共球重叠模型已用于解释正转移值。


























 京公网安备 11010802027423号
京公网安备 11010802027423号