Journal of Advanced Research ( IF 10.7 ) Pub Date : 2022-08-20 , DOI: 10.1016/j.jare.2022.08.008 Qibin Liao 1 , Zhuoqun Liu 2 , Cuisong Zhu 2 , Huan He 2 , Meiqi Feng 2 , Lang Jiang 2 , Xiangqing Ding 2 , Rongxun Sun 3 , Xiaoyan Zhang 2 , Jianqing Xu 2
|
Introduction
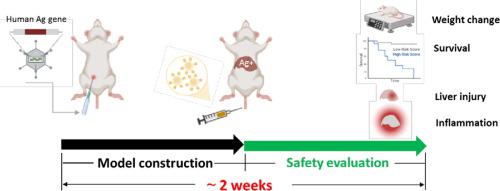
The on-target off-tumor toxicity of chimeric antigen receptor-engineered T cells (CAR-T) might lead to fatal side effects in cancer patients, which remains as a major obstacle to the clinical application of CAR-T immunotherapy. The off-tumor on-target normal tissue toxicity of CAR-T cells needs to be evaluated in preclinical studies using rational animal models.
Objectives
We aim to develop a rational animal model for assessing the off-tumor on-target normal tissue toxicity of various CAR-T cell designs quickly.
Methods
We used a recombinant adenovirus type 5 carrying human HER2/ERBB2 (Ad5-HER2) or CD47 gene (Ad5-CD47) to rapidly generate a mouse model with tunable human antigen expression on normal liver tissue to determine immunotoxicity of traditional CAR-T and hypoxia-response CAR-T cells in vivo.
Results
The obvious liver damage and lymphocyte infiltration were not observed in mice with human antigen-high livers 8 days post-infection. Interestingly, the lethal liver damage, systemic cytokine release and CAR-T cells infiltration in liver were only observed in mice that received traditional CAR-T cells, but not in hypoxia-response CAR-T cells.
Conclusion
Adenovirus-based expression of target antigen in normal mouse tissue may be a useful method for assessing on-target CAR-T cell toxicity in normal tissues, especially various CAR-T cell designs that have the potency of conditional regulation in tumor microenvironment (TME).
中文翻译:

使用复制缺陷型重组腺病毒快速生成用于评估人 CAR-T 细胞靶向正常组织毒性的小鼠模型
介绍
嵌合抗原受体工程化 T 细胞 (CAR-T) 的靶向脱瘤毒性可能导致癌症患者出现致命的副作用,这仍然是 CAR-T 免疫疗法临床应用的主要障碍。CAR-T 细胞的脱瘤靶向正常组织毒性需要在临床前研究中使用合理的动物模型进行评估。
目标
我们的目标是开发一种合理的动物模型,用于快速评估各种 CAR-T 细胞设计的肿瘤外靶向正常组织毒性。
方法
我们使用携带人HER2 / ERBB2 (Ad5-HER2) 或CD47基因 (Ad5-CD47)的重组腺病毒 5 型快速生成在正常肝组织上具有可调人抗原表达的小鼠模型,以确定传统 CAR-T 和缺氧的免疫毒性-体内反应 CAR-T 细胞。
结果
人抗原高肝小鼠感染8天后未见明显肝损伤和淋巴细胞浸润。有趣的是,致死性肝损伤、全身细胞因子释放和 CAR-T 细胞在肝脏中的浸润仅在接受传统 CAR-T 细胞的小鼠中观察到,而在缺氧反应 CAR-T 细胞中没有观察到。
结论
在正常小鼠组织中基于腺病毒的靶抗原表达可能是评估正常组织中靶向 CAR-T 细胞毒性的有用方法,尤其是在肿瘤微环境 (TME) 中具有条件调节能力的各种 CAR-T 细胞设计.


























 京公网安备 11010802027423号
京公网安备 11010802027423号