当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Crystallization-Enabled Henry Reactions: Stereoconvergent Construction of Fully Substituted [N]-Asymmetric Centers
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2022-08-18 , DOI: 10.1021/jacs.2c06669 Pedro De Jesús Cruz 1 , Jeffrey S Johnson 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2022-08-18 , DOI: 10.1021/jacs.2c06669 Pedro De Jesús Cruz 1 , Jeffrey S Johnson 1
Affiliation
|
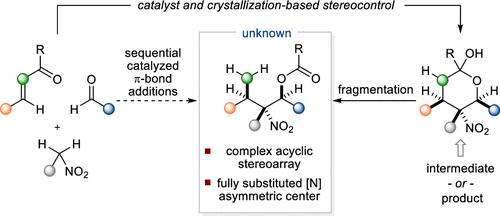
Tetrasubstituted stereogenic carbon centers bearing a nitrogen substituent represent important motifs in medicinal chemistry and natural products; therefore, the development of efficient methods for the stereoselective synthesis of this class of compounds continues to be an important problem. This article describes stereoconvergent Henry reactions of γ,γ-disubstituted nitroalkanes to deliver highly functionalized building blocks containing up to five contiguous stereogenic centers including a fully substituted [N]-asymmetric center. Henry reactions of higher order nitroalkanes are often characterized by their reversibility and minimal accompanying thermodynamic stereocontrol. In contrast, mechanistic studies for the present case suggest a scenario in which reversibility is productively leveraged through crystallization-based stereocontrol, thereby enabling the efficient sequential π-additions of readily accessible starting materials to assemble complex acyclic stereoarrays.
中文翻译:

结晶亨利反应:完全取代的 [N]-不对称中心的立体会聚构建
带有氮取代基的四取代立体碳中心代表了药物化学和天然产物中的重要主题;因此,开发有效的方法来立体选择性合成此类化合物仍然是一个重要的问题。本文描述了 γ,γ-二取代硝基烷烃的立体会聚亨利反应,以提供包含多达五个连续立体中心(包括完全取代的 [N]-不对称中心)的高度功能化的结构单元。高阶硝基烷烃的亨利反应通常以其可逆性和最小的伴随热力学立体控制为特征。相比之下,本案例的机制研究表明,通过基于结晶的立体控制有效地利用可逆性,从而能够对易于获得的起始材料进行有效的顺序π加成,以组装复杂的非循环立体阵列。
更新日期:2022-08-18
中文翻译:

结晶亨利反应:完全取代的 [N]-不对称中心的立体会聚构建
带有氮取代基的四取代立体碳中心代表了药物化学和天然产物中的重要主题;因此,开发有效的方法来立体选择性合成此类化合物仍然是一个重要的问题。本文描述了 γ,γ-二取代硝基烷烃的立体会聚亨利反应,以提供包含多达五个连续立体中心(包括完全取代的 [N]-不对称中心)的高度功能化的结构单元。高阶硝基烷烃的亨利反应通常以其可逆性和最小的伴随热力学立体控制为特征。相比之下,本案例的机制研究表明,通过基于结晶的立体控制有效地利用可逆性,从而能够对易于获得的起始材料进行有效的顺序π加成,以组装复杂的非循环立体阵列。



























 京公网安备 11010802027423号
京公网安备 11010802027423号