Journal of Hepatology ( IF 25.7 ) Pub Date : 2022-08-18 , DOI: 10.1016/j.jhep.2022.07.017 Cheng Tian 1 , Xuewen Min 2 , Yongxu Zhao 1 , Yuchen Wang 1 , Xiaoshan Wu 1 , Situn Liu 1 , Wei Dou 1 , Tingting Zhou 1 , Yan Liu 1 , Rongkui Luo 3 , Zhigang Li 1 , Kathy O Lui 4 , Yu Li 1 , Ben Zhou 1 , Qiurong Ding 5
|
Background & Aims
How hepatic steatosis progresses to non-alcoholic steatohepatitis (NASH) is complicated and remains unclear. The mortality factor 4-like protein 1 (MORF4L1, also called MRG15) was previously identified as a master nuclear chromatin remodeler in the rhythmic regulation of lipid synthesis gene expression in the liver. Whether it also contributes to the progression from liver steatosis to NASH is unclear.
Methods
We adopted 2 different murine NASH models, liver biopsies from patients with NASH, and primary mouse and human hepatocyte cultures for functional examination of MRG15 in NASH progression. Immunoprecipitation-mass spectrometry was applied to identify protein partners of MRG15, and CRISPR targeting was used for gene depletion in liver cells in vivo.
Results
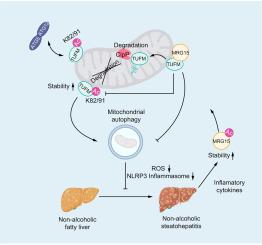
The MRG15 level is increased in the livers of humans and mice with NASH. The inflammatory cytokines in NASH livers stabilize MRG15 by increasing its acetylation. Considerable amounts of MRG15 associate with the outer mitochondrial membrane, where it interacts with and deacetylates the mitochondrial Tu translation elongation factor (TUFM). Deacetylated TUFM, especially at the K82 and K91 sites, is subjected to accelerated degradation by the mitochondrial ClpXP protease system. Reduced liver TUFM consequently results in impaired mitophagy, increased oxidative stress and activation of the NLRP3 inflammasome pathway. Blocking MRG15 expression protects the liver from NASH progression by increasing the stability of liver TUFM. Liver samples from patients with NASH also display a clear reduction in TUFM level, which correlates with increased MRG15 expression.
Conclusion
Collectively, these findings uncover a mitochondrial MRG15-TUFM regulatory pathway that contributes significantly to progression from simple steatosis to NASH, and which could potentially be targeted to treat NASH.
Lay summary
The incidence of non-alcoholic fatty liver disease and its progressive form non-alcoholic steatohepatitis (NASH) is increasing, posing a significant global health challenge. Herein, we have uncovered the importance of the MRG15-TUFM pathway in NASH development. This pathway is active in the mitochondria (energy powerhouse of the cell) and could be targeted for the treatment of NASH.
中文翻译:

MRG15 通过调节 TUFM 的线粒体蛋白水解降解加重非酒精性脂肪性肝炎进展
背景与目标
肝脂肪变性如何进展为非酒精性脂肪性肝炎 (NASH) 是复杂且仍不清楚的。死亡率因子 4 样蛋白 1 (MORF4L1,也称为 MRG15)先前被确定为肝脏脂质合成基因表达节律调节中的主要核染色质重塑剂。它是否也有助于从肝脂肪变性到 NASH 的进展尚不清楚。
方法
我们采用了 2 种不同的小鼠 NASH 模型、来自 NASH 患者的肝活检以及原代小鼠和人类肝细胞培养物,用于 MRG15 在 NASH 进展中的功能检查。免疫沉淀-质谱法用于鉴定 MRG15 的蛋白质伴侣, CRISPR 靶向用于体内肝细胞中的基因耗竭。
结果
患有 NASH 的人类和小鼠肝脏中的 MRG15 水平升高。NASH 肝脏中的炎症细胞因子通过增加其乙酰化来稳定 MRG15。相当数量的 MRG15 与线粒体外膜结合,在那里它与线粒体 Tu 翻译延伸因子 (TUFM)相互作用并使其去乙酰化。脱乙酰化的 TUFM,尤其是在 K82 和 K91 位点,会被线粒体 ClpXP 蛋白酶系统加速降解。肝脏 TUFM 减少因此导致线粒体自噬受损、氧化应激增加和 NLRP3 激活炎性小体途径。阻断 MRG15 表达可通过增加肝脏 TUFM 的稳定性来保护肝脏免于 NASH 进展。来自 NASH 患者的肝脏样本也显示 TUFM 水平明显降低,这与 MRG15 表达增加相关。
结论
总的来说,这些发现揭示了一种线粒体 MRG15-TUFM 调节通路,它对从简单的脂肪变性到 NASH 的进展有显着贡献,并且有可能成为治疗 NASH 的目标。
外行总结
非酒精性脂肪肝病及其进行性非酒精性脂肪性肝炎 (NASH) 的发病率正在增加,对全球健康构成重大挑战。在此,我们揭示了 MRG15-TUFM 通路在 NASH 发展中的重要性。该通路在线粒体(细胞的能量源)中活跃,可作为治疗 NASH 的靶点。


























 京公网安备 11010802027423号
京公网安备 11010802027423号