Structure ( IF 5.7 ) Pub Date : 2022-08-17 , DOI: 10.1016/j.str.2022.07.008 Nozomi Sugano-Nakamura 1 , Kyoko Matoba 1 , Mika Hirose 1 , Nasir K Bashiruddin 2 , Yukiko Matsunaga 1 , Keitaro Yamashita 3 , Kunio Hirata 3 , Masaki Yamamoto 3 , Takao Arimori 1 , Hiroaki Suga 2 , Junichi Takagi 1
|
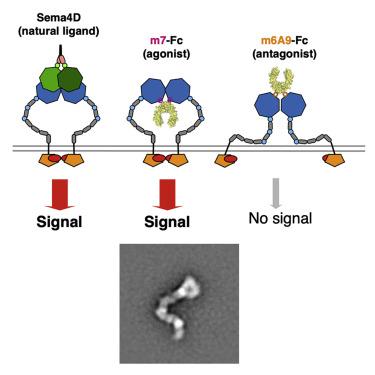
Signaling by single-pass transmembrane receptors often involves a formation of ligand-induced receptor dimers with particular conformation, and bivalent receptor binders can modulate receptor functions by inducing different receptor dimer conformations, although such agents are difficult to design. Here, we describe the generation of both antagonistic and agonistic receptor dimerizers toward PlexinB1 (PlxnB1), a receptor for semaphorin 4D (Sema4D), by grafting two different PlxnB1-binding peptides onto the human immunoglobulin G1 (IgG1) Fc protein. The function-modulating activity of a peptide Fc was strongly dependent on the type of the peptide as well as the grafting site, with the best variants showing activity at an nM concentration range. Structural analysis of each peptide-PlxnB1 complex revealed that the agonistic Fc dimerizes PlxnB1 in a face-to-face fashion similar to that induced by Sema4D, whereas antagonistic Fc would induce signaling-incompetent PlxnB1 dimer conformation, enforcing the idea that plexin activation is primarily controlled by the receptor orientation within the dimer.
中文翻译:

基于 Fc 的从头受体二聚体差异调节 PlexinB1 功能
单程跨膜受体的信号传导通常涉及形成具有特定构象的配体诱导的受体二聚体,并且二价受体结合剂可以通过诱导不同的受体二聚体构象来调节受体功能,尽管此类试剂难以设计。在这里,我们通过将两种不同的 PlxnB1 结合肽移植到人免疫球蛋白 G1 (IgG1) Fc 蛋白上,描述了针对 PlexinB1 (PlxnB1)(一种信号素 4D (Sema4D) 的受体)的拮抗和激动受体二聚体的产生。肽 Fc 的功能调节活性强烈依赖于肽的类型以及移植位点,最佳变体在 nM 浓度范围内显示活性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号