Immunity ( IF 32.4 ) Pub Date : 2022-08-16 , DOI: 10.1016/j.immuni.2022.07.015 Brandon McLeod 1 , Moustafa T Mabrouk 2 , Kazutoyo Miura 3 , Rashmi Ravichandran 4 , Sally Kephart 5 , Sophia Hailemariam 1 , Thao P Pham 3 , Anthony Semesi 6 , Iga Kucharska 6 , Prasun Kundu 6 , Wei-Chiao Huang 2 , Max Johnson 4 , Alyssa Blackstone 4 , Deleah Pettie 4 , Michael Murphy 4 , John C Kraft 4 , Elizabeth M Leaf 4 , Yang Jiao 2 , Marga van de Vegte-Bolmer 7 , Geert-Jan van Gemert 7 , Jordache Ramjith 8 , C Richter King 9 , Randall S MacGill 9 , Yimin Wu 9 , Kelly K Lee 5 , Matthijs M Jore 7 , Neil P King 4 , Jonathan F Lovell 2 , Jean-Philippe Julien 10
|
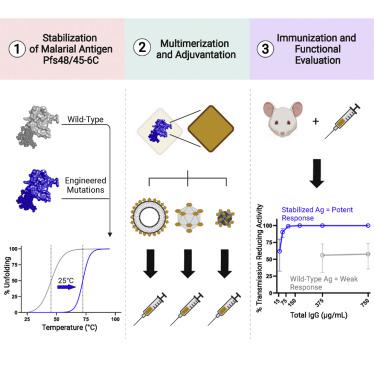
Malaria transmission-blocking vaccines (TBVs) aim to elicit human antibodies that inhibit sporogonic development of Plasmodium falciparum in mosquitoes, thereby preventing onward transmission. Pfs48/45 is a leading clinical TBV candidate antigen and is recognized by the most potent transmission-blocking monoclonal antibody (mAb) yet described; still, clinical development of Pfs48/45 antigens has been hindered, largely by its poor biochemical characteristics. Here, we used structure-based computational approaches to design Pfs48/45 antigens stabilized in the conformation recognized by the most potently inhibitory mAb, achieving >25°C higher thermostability compared with the wild-type protein. Antibodies elicited in mice immunized with these engineered antigens displayed on liposome-based or protein nanoparticle-based vaccine platforms exhibited 1–2 orders of magnitude superior transmission-reducing activity, compared with immunogens bearing the wild-type antigen, driven by improved antibody quality. Our data provide the founding principles for using molecular stabilization solely from antibody structure-function information to drive improved immune responses against a parasitic vaccine target.
中文翻译:

用基于结构的稳定版疟疾抗原 Pfs48/45 接种疫苗可引发超强传播阻断抗体反应
疟疾传播阻断疫苗 (TBV) 旨在引发抑制恶性疟原虫孢子发育的人类抗体在蚊子中,从而防止继续传播。Pfs48/45 是一种领先的临床 TBV 候选抗原,并被迄今为止描述的最有效的传输阻断单克隆抗体 (mAb) 识别;尽管如此,Pfs48/45 抗原的临床开发仍然受到阻碍,主要是由于其生化特性差。在这里,我们使用基于结构的计算方法来设计 Pfs48/45 抗原,该抗原稳定在最有效抑制性 mAb 识别的构象中,与野生型蛋白质相比,实现了 >25°C 的高热稳定性。与携带野生型抗原的免疫原相比,在基于脂质体或基于蛋白质纳米颗粒的疫苗平台上展示的这些工程抗原免疫的小鼠中产生的抗体表现出 1-2 个数量级的传播减少活性,由改进的抗体质量驱动。我们的数据提供了仅使用来自抗体结构-功能信息的分子稳定性来驱动针对寄生虫疫苗靶标的改进免疫反应的基本原则。



























 京公网安备 11010802027423号
京公网安备 11010802027423号