当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Water reduction on the facets of Fe(OH)2: an experimental and DFT study
Environmental Science: Nano ( IF 7.3 ) Pub Date : 2022-08-15 , DOI: 10.1039/d2en00629d Han Song 1 , Xinwen Ou 1 , Mengye Wang 2 , Yan Zhang 3 , Zhang Lin 1, 4, 5
Environmental Science: Nano ( IF 7.3 ) Pub Date : 2022-08-15 , DOI: 10.1039/d2en00629d Han Song 1 , Xinwen Ou 1 , Mengye Wang 2 , Yan Zhang 3 , Zhang Lin 1, 4, 5
Affiliation
|
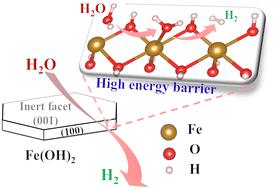
H2 production during the transformation of Fe(OH)2 into magnetite (i.e., Schikorr reaction), has a significant impact on many environmental applications, including the long-term effectiveness of permeable reactive barriers composed of zerovalent iron and the stability of geological radioactive waste repository. However, the reactivity of the Schikorr reaction is not systematically evaluated and its chemical mechanism still remains controversial. Here, we investigate water reduction on the Fe(OH)2 surfaces with the help of an interface-reaction model between water and Fe(OH)2. The Fe(OH)2 hexagonal nanosheets prepared using a coprecipitation method possess dominantly exposed (001) and (100) facets. As unveiled by the DFT simulations, the (001) Fe(OH)2 facet only exposes OH, which is, therefore, inert to water reduction. Moreover, the active Fe-exposed (100) Fe(OH)2 facet is more thermodynamically favourable for water adsorption and dissociation than the (101) and (102) facets. However, the high energy barrier for water dissociation (2.79 eV) may restrict the rate of water reduction on the Fe(OH)2 facet. In addition, the capacity of (100), (101), and (102) Fe(OH)2 facets to adsorb OH− is stronger than that for H2O, which further inhibits water reduction. Furthermore, DOS calculations manifest that Fe d orbitals play a significant role in H2O adsorption, indicating that manipulating the electronic structure of the Fe site is a critical path to mediate water reduction in Fe(OH)2. Our study provides new insights into the understanding of the mechanism of the phase transformation of Fe(OH)2 under anaerobic conditions in environmental and earth sciences.
中文翻译:

Fe(OH)2 表面的水还原:实验和 DFT 研究
Fe(OH) 2转化为磁铁矿(即Schikorr 反应)过程中产生的H 2对许多环境应用具有重大影响,包括零价铁组成的可渗透反应屏障的长期有效性和地质放射性的稳定性废物库。然而,Schikorr 反应的反应性并未得到系统评价,其化学机理仍存在争议。在这里,我们借助水和 Fe(OH) 2之间的界面反应模型研究了 Fe(OH) 2表面的水还原。Fe(OH) 2使用共沉淀法制备的六边形纳米片具有主要暴露的(001)和(100)面。正如 DFT 模拟所揭示的,(001) Fe(OH) 2晶面仅暴露 OH,因此对水的还原是惰性的。此外,与 (101) 和 (102) 晶面相比,活性 Fe 暴露的 (100) Fe(OH) 2晶面在热力学上更利于水的吸附和解离。然而,水离解的高能垒 (2.79 eV) 可能会限制 Fe(OH) 2晶面的水还原速率。此外,(100)、(101)和(102) Fe(OH) 2晶面对OH -的吸附能力强于H 2O,进一步抑制水的减少。此外,DOS计算表明Fe d 轨道在H 2 O吸附中起重要作用,表明操纵Fe位点的电子结构是介导Fe(OH) 2中水还原的关键途径。我们的研究为理解环境和地球科学在厌氧条件下Fe(OH) 2的相变机制提供了新的见解。
更新日期:2022-08-15
中文翻译:

Fe(OH)2 表面的水还原:实验和 DFT 研究
Fe(OH) 2转化为磁铁矿(即Schikorr 反应)过程中产生的H 2对许多环境应用具有重大影响,包括零价铁组成的可渗透反应屏障的长期有效性和地质放射性的稳定性废物库。然而,Schikorr 反应的反应性并未得到系统评价,其化学机理仍存在争议。在这里,我们借助水和 Fe(OH) 2之间的界面反应模型研究了 Fe(OH) 2表面的水还原。Fe(OH) 2使用共沉淀法制备的六边形纳米片具有主要暴露的(001)和(100)面。正如 DFT 模拟所揭示的,(001) Fe(OH) 2晶面仅暴露 OH,因此对水的还原是惰性的。此外,与 (101) 和 (102) 晶面相比,活性 Fe 暴露的 (100) Fe(OH) 2晶面在热力学上更利于水的吸附和解离。然而,水离解的高能垒 (2.79 eV) 可能会限制 Fe(OH) 2晶面的水还原速率。此外,(100)、(101)和(102) Fe(OH) 2晶面对OH -的吸附能力强于H 2O,进一步抑制水的减少。此外,DOS计算表明Fe d 轨道在H 2 O吸附中起重要作用,表明操纵Fe位点的电子结构是介导Fe(OH) 2中水还原的关键途径。我们的研究为理解环境和地球科学在厌氧条件下Fe(OH) 2的相变机制提供了新的见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号