Journal of Allergy and Clinical Immunology ( IF 14.2 ) Pub Date : 2022-08-12 , DOI: 10.1016/j.jaci.2022.07.023 Antonio Bognanni 1 , Derek K Chu 1 , Matthew A Rank 2 , Jonathan Bernstein 3 , Anne K Ellis 4 , David Golden 5 , Matthew Greenhawt 6 , John B Hagan 7 , Caroline C Horner 8 , Dennis K Ledford 9 , Jay Lieberman 10 , Amber U Luong 11 , Lisa A Marks 12 , Richard R Orlandi 13 , Shefali A Samant 14 , Marcus Shaker 15 , Zachary M Soler 16 , Whitney W Stevens 17 , David R Stukus 18 , Julie Wang 19 , Anju T Peters 17
|
Background
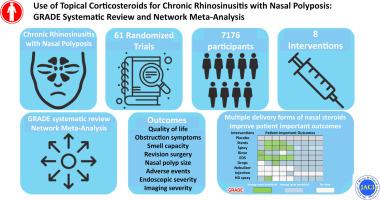
Chronic rhinosinusitis with nasal polyposis (CRSwNP) is associated with a significant disease burden. The optimal use of and administration route for intranasal corticosteroids (INCS) when managing CRSwNP are unclear.
Objective
We systematically synthesized the evidence addressing INCS for CRSwNP.
Methods
We searched studies archived in Medline, Embase, and Central from database inception until September 1, 2021, for randomized controlled trials comparing INCS using any delivery method to placebo or other INCS administration types. Paired reviewers screened records, abstracted data, and rated risk of bias (CLARITY revision of Cochrane Risk of Bias version 1 tool) independently and in duplicate. We synthesized the evidence for each outcome using random effects network meta-analyses. We critically appraised the evidence following the GRADE (Grades of Recommendation Assessment, Development, and Evaluation) approach.
Results
We analyzed 61 randomized controlled trials (7176 participants, 8 interventions). Sinusitis-related quality of life might improve with INCS rinse (mean difference [MD] −6.83, 95% confidence interval [CI] −11.94 to −1.71) and exhalation delivery system (EDS) (MD −7.86, 95% CI −14.64 to −1.08) compared to placebo (both low certainty evidence). Nasal obstruction symptoms are likely improved when receiving INCS via stent/dressing (MD −0.31, 95% CI −0.54 to −0.08), spray (MD −0.51, 95% CI −0.61 to −0.41), and EDS (MD −0.35, 95% CI −0.51 to −0.18) (all moderate to high certainty) compared to placebo. We found no important differences in adverse effects among interventions (moderate certainty for INCS spray, very low to low certainty for others).
Conclusions
Multiple delivery forms of INCS are viable therapeutic options for CRSwNP, resulting in improvement of patient-important outcomes. INCS via stent, spray, and EDS appear to be beneficial across the widest range of considered outcomes.
中文翻译:

外用皮质类固醇治疗伴有鼻息肉的慢性鼻窦炎:GRADE 系统评价和网络荟萃分析
背景
慢性鼻窦炎伴鼻息肉病 (CRSwNP) 与严重的疾病负担相关。管理 CRSwNP 时鼻内皮质类固醇 (INCS) 的最佳使用和给药途径尚不清楚。
客观的
我们系统地综合了针对 CRSwNP 的 INCS 证据。
方法
我们搜索了从数据库开始到 2021 年 9 月 1 日在 Medline、Embase 和 Central 中存档的研究,以寻找将使用任何给药方法的 INCS 与安慰剂或其他 INCS 给药类型进行比较的随机对照试验。配对的审稿人独立并一式两份地筛选记录、抽象数据和偏倚风险评级(Cochrane 偏倚风险工具版本 1 的 CLARITY 修订版)。我们使用随机效应网络荟萃分析综合了每个结果的证据。我们严格按照 GRADE(推荐等级评估、开发和评估)方法对证据进行了评估。
结果
我们分析了 61 项随机对照试验(7176 名参与者,8 项干预措施)。INCS 冲洗(平均差 [MD] -6.83,95% 置信区间 [CI] -11.94 至 -1.71)和呼气输送系统 (EDS)(MD -7.86,95% CI -14.64)可能会改善鼻窦炎相关的生活质量至-1.08)与安慰剂相比(均为低质量证据)。通过支架/敷料(MD -0.31,95% CI -0.54 至 -0.08)、喷雾(MD -0.51,95% CI -0.61 至 -0.41)和 EDS(MD -0.35)接受 INCS 时,鼻塞症状可能会得到改善, 95% CI -0.51 至 -0.18)(均为中度至高度确定性)与安慰剂相比。我们发现干预措施的不良反应没有重要差异(INCS 喷雾的确定性中等,其他确定性非常低至低)。
结论
INCS 的多种递送形式是 CRSwNP 的可行治疗选择,可改善对患者重要的结果。通过支架、喷雾和 EDS 进行的 INCS 似乎在最广泛的考虑结果中都是有益的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号