当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exchange of Adsorbed Pb(II) at the Rutile Surface: Rates and Mechanisms
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2022-08-11 , DOI: 10.1021/acs.est.2c01864 Greg J Ledingham 1 , Weiyi Pan 2 , Daniel E Giammar 2 , Jeffrey G Catalano 1
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2022-08-11 , DOI: 10.1021/acs.est.2c01864 Greg J Ledingham 1 , Weiyi Pan 2 , Daniel E Giammar 2 , Jeffrey G Catalano 1
Affiliation
|
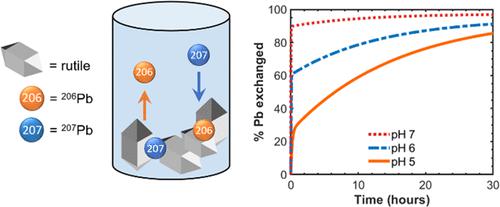
The dynamics of Pb(II) at mineral surfaces affect its mobility in the environment. Pb(II) forms inner- and outer-sphere complexes on mineral surfaces, and this adsorbed pool often represents a large portion of the bioaccessible Pb in contaminated soils. To assess the lability of this potentially reactive adsorbed Pb(II) pool at metal oxide surfaces, we performed Pb(II) isotope exchange measurements between dissolved Pb(II) enriched in 207Pb and natural isotopic abundance Pb(II) adsorbed to rutile at pH 5, 6, and 7. We find that ∼95% of the adsorbed lead is exchangeable. An initially fast exchange (<1 h) is followed by a slower exchange that occurs on a time scale of hours to days. Pb LIII-edge extended X-ray absorption fine structure spectra indicate that similar binding mechanisms are present at all pH values and Pb(II) loadings, implying that differences in exchange rates across the pH range examined are not attributable to changes in the coordination environment. The slower exchange at pH 5 may be associated with interparticle and intraparticle diffusion resulting from particle aggregation. These findings demonstrate that the dissolved Pb(II) pool can be rapidly replenished by adsorbed Pb(II) if this pool is drawn down incrementally by biological uptake or a shift in chemical conditions.
中文翻译:

金红石表面吸附 Pb(II) 的交换:速率和机制
Pb(II) 在矿物表面的动力学影响其在环境中的流动性。Pb(II) 在矿物表面形成内外球复合物,这种吸附池通常代表受污染土壤中生物可及的 Pb 的很大一部分。为了评估金属氧化物表面这种潜在反应性吸附的 Pb(II) 池的不稳定性,我们在富含207 Pb 的溶解 Pb(II) 和吸附在金红石上的天然同位素丰度 Pb(II)之间进行了 Pb(II) 同位素交换测量。 pH 值 5、6 和 7。我们发现 95% 的吸附铅是可交换的。最初的快速交换(<1 小时)之后是在数小时到数天的时间范围内发生的较慢的交换。铅Ⅲ边缘扩展的 X 射线吸收精细结构光谱表明,在所有 pH 值和 Pb(II) 负载下都存在类似的结合机制,这意味着在所检查的 pH 范围内的交换率差异不能归因于配位环境的变化。pH 5 下较慢的交换可能与颗粒聚集导致的颗粒间和颗粒内扩散有关。这些发现表明,如果通过生物吸收或化学条件的变化逐渐减少溶解的 Pb(II) 池,则吸附的 Pb(II) 可以迅速补充溶解的 Pb(II) 池。
更新日期:2022-08-11
中文翻译:

金红石表面吸附 Pb(II) 的交换:速率和机制
Pb(II) 在矿物表面的动力学影响其在环境中的流动性。Pb(II) 在矿物表面形成内外球复合物,这种吸附池通常代表受污染土壤中生物可及的 Pb 的很大一部分。为了评估金属氧化物表面这种潜在反应性吸附的 Pb(II) 池的不稳定性,我们在富含207 Pb 的溶解 Pb(II) 和吸附在金红石上的天然同位素丰度 Pb(II)之间进行了 Pb(II) 同位素交换测量。 pH 值 5、6 和 7。我们发现 95% 的吸附铅是可交换的。最初的快速交换(<1 小时)之后是在数小时到数天的时间范围内发生的较慢的交换。铅Ⅲ边缘扩展的 X 射线吸收精细结构光谱表明,在所有 pH 值和 Pb(II) 负载下都存在类似的结合机制,这意味着在所检查的 pH 范围内的交换率差异不能归因于配位环境的变化。pH 5 下较慢的交换可能与颗粒聚集导致的颗粒间和颗粒内扩散有关。这些发现表明,如果通过生物吸收或化学条件的变化逐渐减少溶解的 Pb(II) 池,则吸附的 Pb(II) 可以迅速补充溶解的 Pb(II) 池。

























 京公网安备 11010802027423号
京公网安备 11010802027423号