当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Balanced Interfacial Ion Concentration and Migration Steric Hindrance Promoting High-Efficiency Deposition/Dissolution Battery Chemistry
Advanced Materials ( IF 29.4 ) Pub Date : 2022-08-11 , DOI: 10.1002/adma.202204681 Zhexuan Liu 1 , Lanyan Li 2 , Liping Qin 3 , Shan Guo 1 , Guozhao Fang 1, 4 , Zhigao Luo 2, 5 , Shuquan Liang 1, 4
Advanced Materials ( IF 29.4 ) Pub Date : 2022-08-11 , DOI: 10.1002/adma.202204681 Zhexuan Liu 1 , Lanyan Li 2 , Liping Qin 3 , Shan Guo 1 , Guozhao Fang 1, 4 , Zhigao Luo 2, 5 , Shuquan Liang 1, 4
Affiliation
|
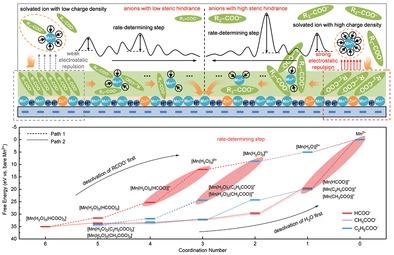
The solid–liquid transition reaction lays the foundation of electrochemical energy storage systems with high capacity, but realizing high efficiency remains a challenge. Herein, in terms of thermodynamics and dynamics, this work demonstrates the significant role of both interfacial H+ concentration and Mn2+ migration steric hindrance for the high-efficiency deposition/dissolution chemistry of zinc–manganese batteries. Specially, the introduction of formate anions can buffer the generated interfacial H+ to stabilize interfacial potential according to the Nernst equation, which stimulates high capacity. Compared with acetate and propionate anions, the formate anion also provides high adsorption density on the cathode surface to shield the electrostatic repulsion due to the small spatial hindrance. Particularly for the solvated Mn2+, the formate-anion-induced lower energy barrier of the rate-determining step during the step-by-step desolvation process results in lower polarization and higher electrochemical reversibility. In situ tests and theoretical calculations verify that the electrolyte with formate anions achieve a good balance between ion concentration and ion-migration steric hindrance. It exhibits both the high energy density of 531.26 W h kg-1 and long cycle life of more than 300 cycles without obvious decay.
中文翻译:

平衡界面离子浓度和迁移空间位阻促进高效沉积/溶解电池化学
固-液转变反应为高容量电化学储能系统奠定了基础,但实现高效率仍然是一个挑战。在此,在热力学和动力学方面,这项工作证明了界面 H +浓度和 Mn 2+迁移空间位阻对锌锰电池高效沉积/溶解化学的重要作用。特别是甲酸根阴离子的引入可以缓冲生成的界面H +根据能斯特方程稳定界面电位,从而激发高容量。与乙酸根和丙酸根阴离子相比,甲酸根阴离子还可以在阴极表面提供高吸附密度,以屏蔽由于空间位阻小而产生的静电排斥。特别是对于溶剂化的 Mn 2+,在逐步去溶剂化过程中,甲酸阴离子诱导的速率决定步骤的较低能垒导致较低的极化和较高的电化学可逆性。原位测试和理论计算验证了含甲酸阴离子的电解质在离子浓度和离子迁移空间位阻之间取得了良好的平衡。它既表现出531.26 Wh kg -1的高能量密度 循环寿命长,循环次数超过300次,无明显衰减。
更新日期:2022-08-11
中文翻译:

平衡界面离子浓度和迁移空间位阻促进高效沉积/溶解电池化学
固-液转变反应为高容量电化学储能系统奠定了基础,但实现高效率仍然是一个挑战。在此,在热力学和动力学方面,这项工作证明了界面 H +浓度和 Mn 2+迁移空间位阻对锌锰电池高效沉积/溶解化学的重要作用。特别是甲酸根阴离子的引入可以缓冲生成的界面H +根据能斯特方程稳定界面电位,从而激发高容量。与乙酸根和丙酸根阴离子相比,甲酸根阴离子还可以在阴极表面提供高吸附密度,以屏蔽由于空间位阻小而产生的静电排斥。特别是对于溶剂化的 Mn 2+,在逐步去溶剂化过程中,甲酸阴离子诱导的速率决定步骤的较低能垒导致较低的极化和较高的电化学可逆性。原位测试和理论计算验证了含甲酸阴离子的电解质在离子浓度和离子迁移空间位阻之间取得了良好的平衡。它既表现出531.26 Wh kg -1的高能量密度 循环寿命长,循环次数超过300次,无明显衰减。

























 京公网安备 11010802027423号
京公网安备 11010802027423号