Journal of Controlled Release ( IF 10.8 ) Pub Date : 2022-08-10 , DOI: 10.1016/j.jconrel.2022.07.043 Hyunsook Kim , Bora Jang , Dayoung Lee , S. Chul Kwon , Hyukjin Lee
|
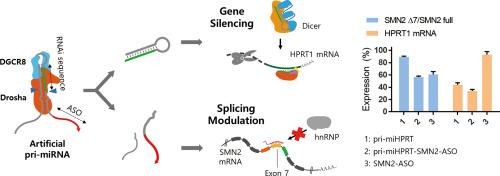
Self-assembled nucleic acid nanostructures have been widely explored for gene therapy applications due to their unique advantages. Their roles are not limited to offer intracellular delivery platforms but additionally provide a biological function to induce targeted gene regulation. Here, we report a self-assembled artificial primary-miRNA (pri-miRNA) for achieving simultaneous multimodal gene regulation. Artificial pri-miRNAs are designed to play a role as substrate RNAs to recruit and interact with Drosha/DGCR8 (Microprocessor). Incorporation of functional RNA motifs and site-specific chemical modification of the primary miRNA are utilized for the biogenesis of two individual gene-regulating oligonucleotides. Once they are cleaved by the endogenous Drosha/DGCR8 complex, basal strands and pre-miRNA can be generated inside of cells. In this study, we integrated basal strands with either SMN2 ASO or anti-miR21 to induce multimodal gene regulation. Microprocessing and subsequent gene regulation were first evaluated by measuring the activity of reporter pre-miRNA. Chemical modification on the primary miRNA was optimized through a series of in vitro Drosha cleavage tests and targeted gene silencing in cells. Primary miRNA with the basal ASO or anti-miR strands showed a successful in vitro activity and resulted in simultaneous multimodal gene regulation in cells. Artificial primary miRNA may offer synergistic therapeutic effects for treating various diseases, including spinal muscular atrophy and cancer.
中文翻译:

人工初级 miRNA 作为同时递送 siRNA 和反义寡核苷酸的平台,用于多模式基因调控
由于其独特的优势,自组装核酸纳米结构已被广泛用于基因治疗应用。它们的作用不仅限于提供细胞内递送平台,还提供诱导靶向基因调控的生物学功能。在这里,我们报告了一种自组装的人工初级 miRNA (pri-miRNA),用于实现同时多模式基因调控。人工 pri-miRNA 旨在发挥作为底物 RNA 的作用,以招募 Drosha/DGCR8(微处理器)并与之相互作用。功能性 RNA 基序的结合和初级 miRNA 的位点特异性化学修饰被用于两个单独的基因调节寡核苷酸的生物发生。一旦它们被内源性 Drosha/DGCR8 复合物切割,就可以在细胞内生成基底链和前 miRNA。在这项研究中,我们将基础链与 SMN2 ASO 或抗 miR21 整合以诱导多模式基因调控。首先通过测量报告基因 pre-miRNA 的活性来评估微处理和随后的基因调控。通过一系列优化对初级 miRNA 的化学修饰体外Drosha 切割测试和细胞中的靶向基因沉默。具有基础 ASO 或抗 miR 链的初级 miRNA 显示出成功的体外活性,并导致细胞中同时进行多模式基因调控。人工初级 miRNA 可以为治疗各种疾病提供协同治疗效果,包括脊髓性肌萎缩和癌症。


























 京公网安备 11010802027423号
京公网安备 11010802027423号