当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
How a Formate Dehydrogenase Responds to Oxygen: Unexpected O2 Insensitivity of an Enzyme Harboring Tungstopterin, Selenocysteine, and [4Fe–4S] Clusters
ACS Catalysis ( IF 12.9 ) Pub Date : 2022-08-10 , DOI: 10.1021/acscatal.2c00316 Joel E. Graham 1 , Dimitri Niks 2 , Grant M. Zane 3 , Qin Gui 3 , Kellie Hom 1 , Russ Hille 2 , Judy D. Wall 3 , C. S. Raman 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2022-08-10 , DOI: 10.1021/acscatal.2c00316 Joel E. Graham 1 , Dimitri Niks 2 , Grant M. Zane 3 , Qin Gui 3 , Kellie Hom 1 , Russ Hille 2 , Judy D. Wall 3 , C. S. Raman 1
Affiliation
|
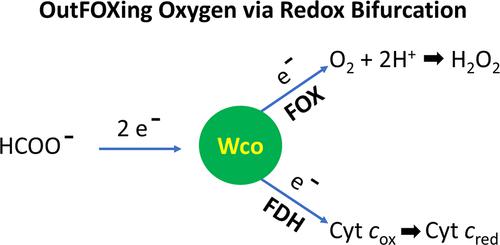
The reversible two-electron interconversion of formate and CO2 is catalyzed by both nonmetallo- and metallo-formate dehydrogenases (FDHs). The latter group comprises molybdenum- or tungsten-containing enzymes with the metal coordinated by two equivalents of a pyranopterin cofactor, a cysteinyl or selenocysteinyl (Sec) ligand supplied by the polypeptide, and a catalytically essential terminal sulfido ligand. In addition, these biocatalysts incorporate one or more [4Fe–4S] clusters for facilitating long-distance electron transfer. However, an interesting dichotomy arises when attempting to understand how the metallo-FDHs react with O2. Whereas existing scholarship portrays these enzymes as being unable to perform in air due to extreme O2 lability of their metal centers, studies dating as far back as the 1930s emphasize that some of these systems exhibit formate oxidase (FOX) activity, coupling formate oxidation to O2 reduction. Therefore, to reconcile these conflicting views, we explored context-dependent functional linkages between metallo-FDHs and their cognate electron acceptors within the same organism vis-à-vis catalysis under atmospheric O2. Here, we report the discovery and characterization of an O2-insensitive FDH2 from the sulfate-reducing bacterium Desulfovibrio vulgaris Hildenborough (DvH) that ligates tungsten, Sec, and four [4Fe–4S] clusters. By advancing a robust expression platform for its recombinant production, we eliminate both the requirement of nitrate or azide during purification and reductive activation with thiols and/or formate prior to catalysis. Because the distinctive spectral signatures of formate-reduced DvH-FDH2 remain invariant under anaerobic and aerobic conditions, we benchmarked the enzyme activity in air, identifying CO2 as the catalytic product. Full reaction progress curve analysis discloses a high catalytic efficiency when probed with a high-potential artificial electron acceptor. Furthermore, we show that DvH-FDH2 enables near-stoichiometric hydrogen peroxide production without superoxide release to achieve O2 insensitivity. Notably, simultaneous electron transfer to cytochrome c and O2 reveals that metal-based electron bifurcation is operational in this system. Taken together, our work proves the co-occurrence of redox bifurcated FDH and FOX activities within a metalloenzyme scaffold. These findings set the stage for uncovering previously unknown O2-insensitive flavin-based electron bifurcation mechanisms, as well as for developing authentic formate/air biofuel cells, engineering O2-stable FDHs and biohybrid metallocatalysts, and discerning formate bioenergetics of gut microbiota.
中文翻译:

甲酸脱氢酶如何对氧气做出反应:含有钨蝶呤、硒代半胱氨酸和 [4Fe-4S] 簇的酶对 O2 的意外不敏感性
甲酸和 CO 2的可逆双电子相互转化由非金属和金属甲酸脱氢酶 (FDH) 催化。后一组包括含钼或钨的酶,其金属由两个当量的吡喃蝶呤辅因子、多肽提供的半胱氨酰或硒代半胱氨酰(Sec)配体和催化必需的末端硫化物配体配位。此外,这些生物催化剂包含一个或多个 [4Fe-4S] 簇,以促进长距离电子转移。然而,当试图了解金属-FDHs 如何与 O 2反应时,会出现一个有趣的二分法。而现有的学术研究将这些酶描述为由于极端的 O 2而无法在空气中发挥作用由于金属中心的不稳定性,早在 1930 年代的研究就强调,其中一些系统表现出甲酸氧化酶 (FOX) 活性,将甲酸氧化与 O 2还原相结合。因此,为了调和这些相互矛盾的观点,我们探索了金属-FDHs 与其同源电子受体在同一生物体内相对于大气 O 2下的催化作用的上下文相关的功能联系。在这里,我们报告了来自硫酸盐还原菌Desulfovibrio vulgaris的 O 2不敏感 FDH2的发现和表征连接钨、Sec 和四个 [4Fe-4S] 簇的 Hildenborough (DvH)。通过为其重组生产推进一个强大的表达平台,我们消除了纯化过程中对硝酸盐或叠氮化物的需求,以及在催化前用硫醇和/或甲酸盐进行还原活化。由于甲酸盐还原的 DvH-FDH2 的独特光谱特征在厌氧和需氧条件下保持不变,我们对空气中的酶活性进行了基准测试,将 CO 2确定为催化产物。全反应进程曲线分析揭示了当用高电位人工电子受体探测时具有高催化效率。此外,我们表明 DvH-FDH2 能够在没有超氧化物释放的情况下实现近化学计量的过氧化氢生产以实现 O 2不敏感。值得注意的是,同时电子转移到细胞色素c和 O 2表明基于金属的电子分叉在该系统中是可操作的。总之,我们的工作证明了金属酶支架内氧化还原分叉的 FDH 和 FOX 活性的共同发生。这些发现为揭示以前未知的 O 2不敏感的基于黄素的电子分叉机制、开发真正的甲酸盐/空气生物燃料电池、工程 O 2稳定的 FDH 和生物混合金属催化剂以及辨别肠道微生物群的甲酸盐生物能学奠定了基础。
更新日期:2022-08-10
中文翻译:

甲酸脱氢酶如何对氧气做出反应:含有钨蝶呤、硒代半胱氨酸和 [4Fe-4S] 簇的酶对 O2 的意外不敏感性
甲酸和 CO 2的可逆双电子相互转化由非金属和金属甲酸脱氢酶 (FDH) 催化。后一组包括含钼或钨的酶,其金属由两个当量的吡喃蝶呤辅因子、多肽提供的半胱氨酰或硒代半胱氨酰(Sec)配体和催化必需的末端硫化物配体配位。此外,这些生物催化剂包含一个或多个 [4Fe-4S] 簇,以促进长距离电子转移。然而,当试图了解金属-FDHs 如何与 O 2反应时,会出现一个有趣的二分法。而现有的学术研究将这些酶描述为由于极端的 O 2而无法在空气中发挥作用由于金属中心的不稳定性,早在 1930 年代的研究就强调,其中一些系统表现出甲酸氧化酶 (FOX) 活性,将甲酸氧化与 O 2还原相结合。因此,为了调和这些相互矛盾的观点,我们探索了金属-FDHs 与其同源电子受体在同一生物体内相对于大气 O 2下的催化作用的上下文相关的功能联系。在这里,我们报告了来自硫酸盐还原菌Desulfovibrio vulgaris的 O 2不敏感 FDH2的发现和表征连接钨、Sec 和四个 [4Fe-4S] 簇的 Hildenborough (DvH)。通过为其重组生产推进一个强大的表达平台,我们消除了纯化过程中对硝酸盐或叠氮化物的需求,以及在催化前用硫醇和/或甲酸盐进行还原活化。由于甲酸盐还原的 DvH-FDH2 的独特光谱特征在厌氧和需氧条件下保持不变,我们对空气中的酶活性进行了基准测试,将 CO 2确定为催化产物。全反应进程曲线分析揭示了当用高电位人工电子受体探测时具有高催化效率。此外,我们表明 DvH-FDH2 能够在没有超氧化物释放的情况下实现近化学计量的过氧化氢生产以实现 O 2不敏感。值得注意的是,同时电子转移到细胞色素c和 O 2表明基于金属的电子分叉在该系统中是可操作的。总之,我们的工作证明了金属酶支架内氧化还原分叉的 FDH 和 FOX 活性的共同发生。这些发现为揭示以前未知的 O 2不敏感的基于黄素的电子分叉机制、开发真正的甲酸盐/空气生物燃料电池、工程 O 2稳定的 FDH 和生物混合金属催化剂以及辨别肠道微生物群的甲酸盐生物能学奠定了基础。

























 京公网安备 11010802027423号
京公网安备 11010802027423号