当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rhodium(III)-Catalyzed Redox-Neutral [4 + 1]-Annulation of Unactivated Alkenes with Sulfoxonium Ylides
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2022-08-10 , DOI: 10.1021/acs.joc.2c01324 Pinki Sihag 1 , Masilamani Jeganmohan 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2022-08-10 , DOI: 10.1021/acs.joc.2c01324 Pinki Sihag 1 , Masilamani Jeganmohan 1
Affiliation
|
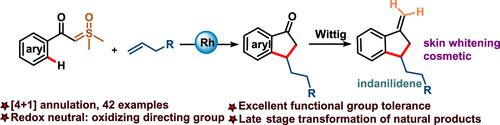
A novel methodology for redox-neutral [4 + 1] annulation of unactivated alkenes with sulfoxonium ylides leads to the synthesis of a diverse library of indanone compounds. The developed annulation reaction was found to be highly versatile due to its compatibility with various unactivated alkenes functionalized with various sensitive functional groups as well as substituted sulfoxonium ylides. Further, multiple transformations such as ring-expansion, reduction, aldol condensation, and Wittig reaction were carried out with indanones. Using this way, highly useful cyclic heterocycles such as indene, dihydroisocoumarin, and 1-indanilidene were prepared in a single step. A possible reaction mechanism was supported by deuterium labeling studies, competitive studies, and kinetic isotopic studies.
中文翻译:

铑 (III)-催化氧化还原-中性 [4 + 1]-未活化烯烃与硫鎓叶立德的环化
一种用于氧化还原中性 [4 + 1] 环化未活化烯烃与氧化鎓叶立德的新方法导致合成了多种茚满酮化合物库。开发的环化反应被发现具有高度通用性,因为它与各种用各种敏感官能团功能化的未活化烯烃以及取代的氧化鎓叶立德相容。此外,茚满酮还进行了扩环、还原、羟醛缩合和维蒂希反应等多种转化。使用这种方法,在一个步骤中制备了非常有用的环状杂环,例如茚、二氢异香豆素和 1-茚满。氘标记研究、竞争研究和动力学同位素研究支持了一种可能的反应机制。
更新日期:2022-08-10
中文翻译:

铑 (III)-催化氧化还原-中性 [4 + 1]-未活化烯烃与硫鎓叶立德的环化
一种用于氧化还原中性 [4 + 1] 环化未活化烯烃与氧化鎓叶立德的新方法导致合成了多种茚满酮化合物库。开发的环化反应被发现具有高度通用性,因为它与各种用各种敏感官能团功能化的未活化烯烃以及取代的氧化鎓叶立德相容。此外,茚满酮还进行了扩环、还原、羟醛缩合和维蒂希反应等多种转化。使用这种方法,在一个步骤中制备了非常有用的环状杂环,例如茚、二氢异香豆素和 1-茚满。氘标记研究、竞争研究和动力学同位素研究支持了一种可能的反应机制。


























 京公网安备 11010802027423号
京公网安备 11010802027423号