European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2022-08-08 , DOI: 10.1016/j.ejmech.2022.114647 Lianqi Sun 1 , Shuo Zhang 1 , Shibo Kou 1 , Hong Yi 1 , Along Cui 1 , Zhuorong Li 1
|
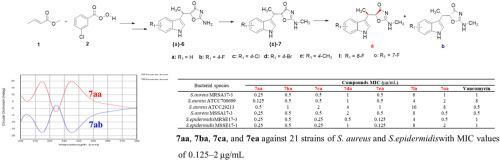
In this study, indlomycin, an inhibitor of tryptophanyl-tRNA synthetase (TrpRS), and 29 racemic indolmycin derivatives were synthesized, their antibacterial activity were evaluated against methicillin-resistant Staphylococcus aureus (S. aureus) NRS384, ATCC29213, and Escherichia coli (E. coli) ATCC25922 strains. Compounds (±)-7a, (±)-7b, (±)-7c and (±)-7e exhibited minimum inhibitory concentration (MIC) values of 1–2 μg/mL against S. aureus NRS384 and ATCC29213, exhibiting significant antibacterial activity, but none of the compounds exhibited antibacterial activity against E. coli. To investigate the effect of conformation on antibacterial activity, seven racemic compounds with good antibacterial activity were separated, and the antibacterial activity of these 14 compounds was evaluated on 25 bacterial strains. This revealed that the isomers with natural conformations (1′R, 5S) had significantly better antibacterial activity than the enantiomeric isomers and racemates. Compounds 7aa, 7ba, 7ca, and 7ea exhibited good antibacterial activity against 21 strains of S. aureus and S. epidermidis with MIC values of 0.125–2 μg/mL, which were superior to that of vancomycin, used in clinical practice. The compounds 7aa, 7ba, 7ca and 7ea were moderately bound to plasma proteins and were stable in the whole blood of CD-1 mice. In conclusion, a series of new indomycin derivatives with stronger antibacterial activity against G+ bacteria were obtained.
中文翻译:

色氨酸-tRNA合成酶抑制剂吲哚霉素衍生物的设计、合成及抗菌活性
本研究合成了一种色氨酸-tRNA合成酶(TrpRS)抑制剂吲哚霉素和29种消旋吲哚霉素衍生物,评估了它们对耐甲氧西林金黄色葡萄球菌(S. aureus)NRS384、ATCC29213和大肠杆菌( Escherichia coli)的抗菌活性。 . 大肠杆菌) ATCC25922 菌株。化合物( ± ) -7a 、( ± )-7b、( ± )-7c和( ± )-7e对金黄色葡萄球菌的最小抑菌浓度 (MIC) 值为 1–2 μg/mLNRS384 和 ATCC29213 表现出显着的抗菌活性,但没有一种化合物表现出对大肠杆菌的抗菌活性。为了研究构象对抗菌活性的影响,分离出7种具有良好抗菌活性的外消旋化合物,并对这14种化合物对25株细菌的抗菌活性进行了评价。这表明具有天然构象的异构体(1'R,5S)比对映异构体和外消旋体具有显着更好的抗菌活性。化合物7aa , 7ba , 7ca , 7ea对21株金黄色葡萄球菌表现出良好的抗菌活性和表皮葡萄球菌,MIC值为0.125-2 μg/mL,优于万古霉素,用于临床。化合物7aa、7ba、7ca和7ea与血浆蛋白适度结合,在CD-1小鼠全血中稳定。综上所述,获得了一系列对G +菌具有更强抗菌活性的新型吲哚霉素衍生物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号