当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cross-linked proton-exchange membranes with strongly reduced fuel crossover and increased chemical stability for direct-isopropanol fuel cells
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2022-08-09 , DOI: 10.1039/d2ta03832c Sebastian Auffarth 1, 2 , Willibald Dafinger 1, 2 , Julia Mehler 1 , Valeria Ardizzon 1, 2 , Patrick Preuster 1 , Peter Wasserscheid 1, 3 , Simon Thiele 1, 2 , Jochen Kerres 1, 4
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2022-08-09 , DOI: 10.1039/d2ta03832c Sebastian Auffarth 1, 2 , Willibald Dafinger 1, 2 , Julia Mehler 1 , Valeria Ardizzon 1, 2 , Patrick Preuster 1 , Peter Wasserscheid 1, 3 , Simon Thiele 1, 2 , Jochen Kerres 1, 4
Affiliation
|
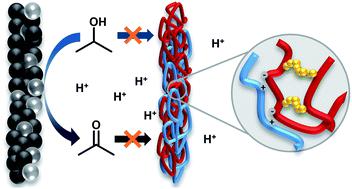
Isopropanol fuel cells offer an attractive way to provide electric energy from a liquid, easily transportable fuel without producing significant amounts of CO2. The oxidation product acetone can be easily hydrogenated back to isopropanol to close the storage cycle, thereby avoiding the sophisticated handling of fugitive molecular hydrogen. Until now, direct-isopropanol fuel cells (DIFC) usually rely on various perfluorosulfonic acid ionomers, like Nafion, which are costly and have an unfavorable high fluorine content. Additionally, the dissolution of Nafion in isopropanol/acetone/water solutions within respective applications has prevented the long time operation of DIFCs so far. The swelling of those ionomers during operation promotes fuel crossover and reduces the system's overall energy efficiency. This study uses ionic cross-linking of polymer blends to manufacture chemically stable membranes and introduces a new click-like covalent cross-linking strategy for ion exchange polymers. Compared to Nafion XL, the manufactured membranes increase the maximum power density by up to 10%, resist a dissolution stress test up to 84 w% and reduce the detected isopropanol/acetone crossover up to 75/100% during fuel cell operation. Consequently, the material can be considered a major step toward the technical implementation of isopropanol fuel cell technologies.
中文翻译:

用于直接异丙醇燃料电池的交联质子交换膜可大大降低燃料交叉并提高化学稳定性
异丙醇燃料电池提供了一种有吸引力的方法,可以从液体、易于运输的燃料中提供电能,而不会产生大量的 CO 2. 氧化产物丙酮可以很容易地加氢回异丙醇以关闭储存循环,从而避免对逃逸分子氢的复杂处理。到目前为止,直接异丙醇燃料电池 (DIFC) 通常依赖于各种全氟磺酸离聚物,例如 Nafion,这些离聚物成本高且含氟量高。此外,迄今为止,Nafion 在各自应用中溶解在异丙醇/丙酮/水溶液中,阻碍了 DIFC 的长时间运行。这些离聚物在运行过程中的膨胀会促进燃料交叉并降低系统的整体能源效率。本研究使用聚合物共混物的离子交联来制造化学稳定的膜,并为离子交换聚合物引入了一种新的点击式共价交联策略。与 Nafion XL 相比,所制造的膜将最大功率密度提高了 10%,在燃料电池运行期间可抵抗高达 84 w% 的溶解应力测试,并将检测到的异丙醇/丙酮交叉降低高达 75/100%。因此,该材料可被视为异丙醇燃料电池技术的技术实施迈出的重要一步。
更新日期:2022-08-09
中文翻译:

用于直接异丙醇燃料电池的交联质子交换膜可大大降低燃料交叉并提高化学稳定性
异丙醇燃料电池提供了一种有吸引力的方法,可以从液体、易于运输的燃料中提供电能,而不会产生大量的 CO 2. 氧化产物丙酮可以很容易地加氢回异丙醇以关闭储存循环,从而避免对逃逸分子氢的复杂处理。到目前为止,直接异丙醇燃料电池 (DIFC) 通常依赖于各种全氟磺酸离聚物,例如 Nafion,这些离聚物成本高且含氟量高。此外,迄今为止,Nafion 在各自应用中溶解在异丙醇/丙酮/水溶液中,阻碍了 DIFC 的长时间运行。这些离聚物在运行过程中的膨胀会促进燃料交叉并降低系统的整体能源效率。本研究使用聚合物共混物的离子交联来制造化学稳定的膜,并为离子交换聚合物引入了一种新的点击式共价交联策略。与 Nafion XL 相比,所制造的膜将最大功率密度提高了 10%,在燃料电池运行期间可抵抗高达 84 w% 的溶解应力测试,并将检测到的异丙醇/丙酮交叉降低高达 75/100%。因此,该材料可被视为异丙醇燃料电池技术的技术实施迈出的重要一步。


























 京公网安备 11010802027423号
京公网安备 11010802027423号