当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Passivation of Cu nanosheet dissolution with Cu2+-containing electrolytes for selective electroreduction of CO2 to CH4
Environmental Science: Nano ( IF 7.3 ) Pub Date : 2022-08-08 , DOI: 10.1039/d2en00561a Guozhu Chen 1 , Junwei Fu 1 , Bao Liu 1 , Chao Cai 1 , Hongmei Li 1, 2 , Zhibin Zhang 3 , Kaihui Liu 3 , Zhang Lin 4 , Min Liu 1
Environmental Science: Nano ( IF 7.3 ) Pub Date : 2022-08-08 , DOI: 10.1039/d2en00561a Guozhu Chen 1 , Junwei Fu 1 , Bao Liu 1 , Chao Cai 1 , Hongmei Li 1, 2 , Zhibin Zhang 3 , Kaihui Liu 3 , Zhang Lin 4 , Min Liu 1
Affiliation
|
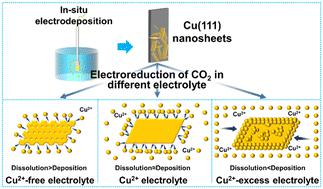
Electrocatalytic conversion of carbon dioxide (CO2) to methane (CH4) has drawn great attention for solving the problem of global warming by reducing greenhouse gases and recycling carbon resources. Among reported Cu-based catalysts, Cu nanosheets (Cu NSs) with a highly exposed (111) facet are beneficial for converting CO2 to CH4. However, the stability issue of Cu NSs, due to their dissolution, severely limits their performance and practical application in electrocatalytic CO2 reduction. Here, we report a method to effectively passivate the dissolution of Cu NSs by adding an appropriate amount of Cu2+ in the electrolyte, which improves the selectivity and stability of CO2 reduction to CH4. Structural characterization and CO2 reduction tests indicate that the Cu NSs maintain the exposed (111) facet and thus maintain the efficient reduction of CO2 to CH4 in the electrolyte containing an appropriate Cu2+ concentration. The Cu NSs exhibit a high CH4 selectivity of ∼70% and a high stability of ∼60% for ∼10 h. This work indicates that a moderate amount of Cu2+ in the electrolyte can passivate the Cu dissolution and increase the stability of Cu electrodes, providing a new perspective for achieving better stability of catalysts in the future.
中文翻译:

用含 Cu2+ 的电解质钝化 Cu 纳米片溶解以将 CO2 选择性电还原为 CH4
将二氧化碳(CO 2)电催化转化为甲烷(CH 4),通过减少温室气体排放和循环利用碳资源来解决全球变暖问题引起了人们的极大关注。在已报道的铜基催化剂中,具有高度暴露 (111) 晶面的铜纳米片 (Cu NSs) 有利于将 CO 2转化为 CH 4。然而,由于Cu NSs的溶解性问题,其稳定性问题严重限制了其在电催化CO 2还原中的性能和实际应用。在这里,我们报道了一种通过在电解液中添加适量的 Cu 2+来有效钝化 Cu NSs 溶解的方法,该方法提高了 CO 的选择性和稳定性。2还原为 CH 4。结构表征和CO 2还原测试表明,Cu NSs 保持了暴露的(111) 晶面,因此在含有适当Cu 2+浓度的电解液中保持了CO 2到CH 4的有效还原。Cu NSs表现出~70%的高CH 4选择性和~60%的高稳定性~10小时。该工作表明,电解液中适量的Cu 2+可以钝化Cu的溶解,提高Cu电极的稳定性,为未来获得更好的催化剂稳定性提供了新的视角。
更新日期:2022-08-08
中文翻译:

用含 Cu2+ 的电解质钝化 Cu 纳米片溶解以将 CO2 选择性电还原为 CH4
将二氧化碳(CO 2)电催化转化为甲烷(CH 4),通过减少温室气体排放和循环利用碳资源来解决全球变暖问题引起了人们的极大关注。在已报道的铜基催化剂中,具有高度暴露 (111) 晶面的铜纳米片 (Cu NSs) 有利于将 CO 2转化为 CH 4。然而,由于Cu NSs的溶解性问题,其稳定性问题严重限制了其在电催化CO 2还原中的性能和实际应用。在这里,我们报道了一种通过在电解液中添加适量的 Cu 2+来有效钝化 Cu NSs 溶解的方法,该方法提高了 CO 的选择性和稳定性。2还原为 CH 4。结构表征和CO 2还原测试表明,Cu NSs 保持了暴露的(111) 晶面,因此在含有适当Cu 2+浓度的电解液中保持了CO 2到CH 4的有效还原。Cu NSs表现出~70%的高CH 4选择性和~60%的高稳定性~10小时。该工作表明,电解液中适量的Cu 2+可以钝化Cu的溶解,提高Cu电极的稳定性,为未来获得更好的催化剂稳定性提供了新的视角。


























 京公网安备 11010802027423号
京公网安备 11010802027423号