当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Carboxamide-Directed Stereospecific Couplings of Chiral Tertiary Alkyl Halides with Terminal Alkynes
ACS Catalysis ( IF 12.9 ) Pub Date : 2022-08-08 , DOI: 10.1021/acscatal.2c02433 Hiroki Akagawa 1 , Naoki Tsuchiya 1 , Asuka Morinaga 1 , Yu Katayama 1 , Michinori Sumimoto 1 , Takashi Nishikata 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2022-08-08 , DOI: 10.1021/acscatal.2c02433 Hiroki Akagawa 1 , Naoki Tsuchiya 1 , Asuka Morinaga 1 , Yu Katayama 1 , Michinori Sumimoto 1 , Takashi Nishikata 1
Affiliation
|
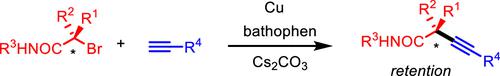
Herein, we report the stereospecific Sonogashira coupling of a chiral α-bromocarboxamide possessing a tert-alkyl moiety and an alkyne; this reaction produces a chiral tert-alkylated alkyne in a stereoretentive manner. In this reaction, both the CuBr/bathophen catalyst system and carboxamide directing group are essential for achieving the high enantiospecificities of the couplings. Mechanistic studies of this reaction revealed that the alkynyl copper species is the key intermediate that coordinates to the carboxamide group of chiral α-bromocarboxamide.
中文翻译:

手性叔烷基卤化物与末端炔烃的羧酰胺定向立体特异性偶联
在此,我们报道了具有叔烷基部分和炔烃的手性 α-溴代甲酰胺的立体定向 Sonogashira 偶联。该反应以立体保持方式产生手性叔烷基化炔烃。在该反应中,CuBr/bathophen 催化剂体系和羧酰胺导向基团对于实现偶联的高对映体特异性都是必不可少的。对该反应的机理研究表明,炔基铜是与手性 α-溴代甲酰胺的甲酰胺基团配位的关键中间体。
更新日期:2022-08-08
中文翻译:

手性叔烷基卤化物与末端炔烃的羧酰胺定向立体特异性偶联
在此,我们报道了具有叔烷基部分和炔烃的手性 α-溴代甲酰胺的立体定向 Sonogashira 偶联。该反应以立体保持方式产生手性叔烷基化炔烃。在该反应中,CuBr/bathophen 催化剂体系和羧酰胺导向基团对于实现偶联的高对映体特异性都是必不可少的。对该反应的机理研究表明,炔基铜是与手性 α-溴代甲酰胺的甲酰胺基团配位的关键中间体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号