当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of Open-Cage Fullerenes Containing a H-Bond between the Encapsulated Water Molecule and the Amide Moiety on the Rim of the Orifice
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2022-08-01 , DOI: 10.1002/ejoc.202200854 Zhen Liu 1 , Rui Gao 1 , Zeyu Liu 1 , Hong Fei Han 1 , Jie Su 1 , Peng Jin 2 , Liangbing Gan 3
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2022-08-01 , DOI: 10.1002/ejoc.202200854 Zhen Liu 1 , Rui Gao 1 , Zeyu Liu 1 , Hong Fei Han 1 , Jie Su 1 , Peng Jin 2 , Liangbing Gan 3
Affiliation
|
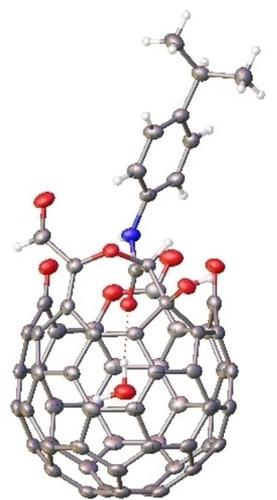
Quantitative water encapsulation is reached during the formation process of the open-cage fullerene with a 17-member orifice which is completely blocked by the amide group directly above the orifice. The amide oxygen forms a H-bond with the trapped water. The bond length (O⋅⋅⋅H−O) is 2.859 Å as shown by single crystal X-ray analysis, and the H-bond energy is about −3.83 kcal/mol by theoretical calculation.
中文翻译:

包封水分子与孔口边缘酰胺部分之间含有氢键的开笼富勒烯的合成
在具有 17 元孔的开笼富勒烯的形成过程中实现了定量的水包封,该 17 元孔被直接位于孔上方的酰胺基团完全阻挡。酰胺氧与捕获的水形成氢键。单晶X射线分析表明键长(O···H-O)为2.859 Å,理论计算H键能量约为-3.83 kcal/mol。
更新日期:2022-08-01
中文翻译:

包封水分子与孔口边缘酰胺部分之间含有氢键的开笼富勒烯的合成
在具有 17 元孔的开笼富勒烯的形成过程中实现了定量的水包封,该 17 元孔被直接位于孔上方的酰胺基团完全阻挡。酰胺氧与捕获的水形成氢键。单晶X射线分析表明键长(O···H-O)为2.859 Å,理论计算H键能量约为-3.83 kcal/mol。



























 京公网安备 11010802027423号
京公网安备 11010802027423号