Clinical Gastroenterology and Hepatology ( IF 12.6 ) Pub Date : 2022-08-06 , DOI: 10.1016/j.cgh.2022.07.032 Virginia Solitano 1 , Antonio Facciorusso 2 , Tine Jess 3 , Christopher Ma 4 , Cesare Hassan 5 , Alessandro Repici 5 , Vipul Jairath 6 , Alessandro Armuzzi 5 , Siddharth Singh 7
|
Background & Aims
Safety is a key consideration when choosing advanced therapies (biologic agents and oral small-molecule inhibitors/modulators) in patients with inflammatory bowel diseases (IBDs). We performed a systematic review and meta-analysis comparing the risk of serious infections with advanced therapies in active comparator studies.
Methods
Through a systematic search until February 28, 2022, we included 20 head-to-head studies comparing risk of serious infections with tumor necrosis factor α (TNFα) antagonists, vedolizumab, ustekinumab, tofacitinib, filgotinib, and ozanimod in patients with IBD. We performed random-effects meta-analysis comparing different advanced therapies.
Results
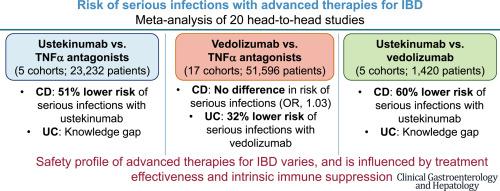
No significant difference was observed in the risk of serious infections between vedolizumab vs TNFα antagonists in all patients with IBD (17 cohorts: odds ratio [OR], 0.84; 95% CI, 0.68–1.04), with moderate heterogeneity (I2 = 37%); on subgroup analysis, vedolizumab was associated with a lower risk of serious infections in patients with ulcerative colitis (11 cohorts: OR, 0.68; 95% CI, 0.56–0.83; I2 = 0%), but not in Crohn’s disease (CD) (9 cohorts: OR, 1.03; 95% CI, 0.78–1.35; I2 = 42%). Age, sex, prior biologic exposure, and use of biologic monotherapy did not influence this association. In patients with CD, ustekinumab was associated with a lower risk of serious infections vs TNFα antagonists (3 cohorts: OR, 0.49; 95% CI, 0.25–0.93; I2 = 16%) and vs vedolizumab (3 cohorts: OR, 0.40; 95% CI, 0.17–0.93; I2 = 67%). Few studies compared other advanced therapies.
Conclusions
Vedolizumab may offer net benefit over TNFα antagonists in patients with ulcerative colitis, but not in CD. Ustekinumab may offer net benefit over TNFα antagonists and vedolizumab in patients with CD.
中文翻译:

炎症性肠病中生物制剂和口服小分子药物严重感染的比较风险:系统评价和荟萃分析
背景与目标
在为炎症性肠病 (IBD) 患者选择先进疗法(生物制剂和口服小分子抑制剂/调节剂)时,安全性是一个关键考虑因素。我们进行了系统回顾和荟萃分析,比较了主动比较研究中的严重感染风险和先进疗法。
方法
通过截至 2022 年 2 月 28 日的系统检索,我们纳入了 20 项头对头研究,比较肿瘤坏死因子 α (TNFα) 拮抗剂、维多珠单抗、乌特克单抗、托法替尼、非戈替尼和奥扎莫德在 IBD 患者中发生严重感染的风险。我们进行了比较不同先进疗法的随机效应荟萃分析。
结果
在所有 IBD 患者中,维多珠单抗与 TNFα 拮抗剂之间的严重感染风险没有观察到显着差异(17 个队列:比值比 [OR],0.84;95% CI,0.68–1.04),具有中等异质性(I 2 = 37 ) %); 亚组分析显示,维多珠单抗与溃疡性结肠炎患者严重感染风险较低相关(11 个队列:OR,0.68;95% CI,0.56–0.83;I 2 = 0%),但与克罗恩病 (CD ) 无关(9 个队列:OR,1.03;95% CI,0.78–1.35;I 2 = 42%)。年龄、性别、既往生物制剂暴露和生物单一疗法的使用不影响这种关联。在 CD 患者中,与 TNFα 拮抗剂(3 个队列:OR,0.49;95% CI,0.25–0.93;I 2 = 16%)和维多珠单抗(3 个队列:OR,0.40)相比,乌特克单抗与较低的严重感染风险相关;95% CI,0.17–0.93;I 2 = 67%)。很少有研究比较其他先进疗法。
结论
对于溃疡性结肠炎患者,维多珠单抗可能比 TNFα 拮抗剂提供净益处,但对于 CD 患者则不然。在 CD 患者中,乌司奴单抗可能比 TNFα 拮抗剂和维多珠单抗带来净效益。


























 京公网安备 11010802027423号
京公网安备 11010802027423号