Journal of Cleaner Production ( IF 11.1 ) Pub Date : 2022-08-05 , DOI: 10.1016/j.jclepro.2022.133402 Taka-Aki Shinozaki , Masahiko Suenaga , Yohan Ko , Eiji Yamamoto , Haruno Murayama , Makoto Tokunaga
|
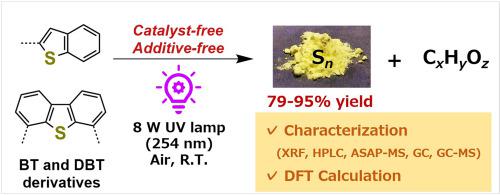
When producing liquid fuels from petroleum, hydrodesulfurization methods reduce the concentration of sulfur to ≤10 mg(S)/L (weight/volume concentration of sulfur), namely, ≤ 0.31 mmol/L. Dibenzothiophene derivatives (DBTs), which are known as particularly difficult desulfurizing substances, have been decomposed reductively in severe conditions of high temperatures (270–372 °C) and high pressure (50–102 atm of H2). In this study, we developed a UV light irradiation-based desulfurization method for aromatic sulfur compounds such as benzothiophene derivatives (BTs) and DBTs under room temperature and atmospheric pressure without the use of catalysts or additives. This method is simple, location-independent, and low-cost, and has low environmental impact. BTs and DBTs completely decomposed in approximately 8 h and 16 h, respectively, under ultraviolet (UV) light irradiation (λ = 254 nm) from a 8 W lamp. The yellow precipitates that were produced upon decomposition were confirmed to be sulfur allotropes (Sn). The residual hydrocarbon portion of DBT after sulfur removal was determined to be benzene. The decomposition reaction was determined to exhibit pseudo-first-order reaction. DFT calculations confirmed the degradation mechanism as follows: UV light irradiation induces a photochemically excited triplet state of DBTs. The excited DBTs reacts with O2 to form a π-complex, which isomerizes to a more stable σ-complex. The DBTs-O2 (σ-complex) then reacts with free DBTs to afford two molecules of dibenzothiophene-5-oxide derivatives (DBTOs), which are excited to singlet states on photoirradiation. The excited DBTOs isomerize through a minimum energy intersection eventually to dibenzofuran episulfides from which sulfur extrusion occurs.
中文翻译:

紫外光诱导苯并噻吩和二苯并噻吩衍生物分解,无需添加剂和催化剂即可有效脱硫
用石油生产液体燃料时,加氢脱硫方法将硫的浓度降低到≤10 mg(S)/L(硫的重量/体积浓度),即≤0.31 mmol/L。二苯并噻吩衍生物 (DBTs) 被称为特别难脱硫的物质,在高温(270–372 °C)和高压(50–102 atm H2)的苛刻条件下会发生还原性分解。在这项研究中,我们开发了一种基于紫外光照射的芳香族硫化合物如苯并噻吩衍生物(BTs)和 DBTs 在室温和大气压下不使用催化剂或添加剂的脱硫方法。该方法简单、位置无关、成本低,且具有环境影响小。 在8 W 灯的紫外 (UV) 光照射 ( λ = 254 nm)下,BT 和 DBT 分别在大约 8 小时和 16 小时内完全分解。分解时产生的黄色沉淀物被确认为硫同素异形体(S n )。脱硫后 DBT 的残留烃部分被确定为苯。分解反应被确定为表现出准一级反应。DFT计算证实了降解机制如下:紫外光照射诱导DBTs的光化学激发三重态。激发的 DBT 与 O 2反应形成 π-配合物,其异构化为更稳定的 σ-配合物。DBTs-O 2(σ-络合物)然后与游离 DBT 反应,得到两个分子的二苯并噻吩-5-氧化物衍生物 (DBTO),它们在光辐照下被激发成单重态。激发的 DBTO 通过最小能量交叉最终异构化为二苯并呋喃环硫化物,从中发生硫挤出。



























 京公网安备 11010802027423号
京公网安备 11010802027423号