当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photoinduced Oxygen Transfer Using Nitroarenes for the Anaerobic Cleavage of Alkenes
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2022-08-05 , DOI: 10.1021/jacs.2c05648 Dan E Wise 1 , Emma S Gogarnoiu 1 , Alana D Duke 1 , Joshua M Paolillo 1 , Taylor L Vacala 1 , Waseem A Hussain 1 , Marvin Parasram 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2022-08-05 , DOI: 10.1021/jacs.2c05648 Dan E Wise 1 , Emma S Gogarnoiu 1 , Alana D Duke 1 , Joshua M Paolillo 1 , Taylor L Vacala 1 , Waseem A Hussain 1 , Marvin Parasram 1
Affiliation
|
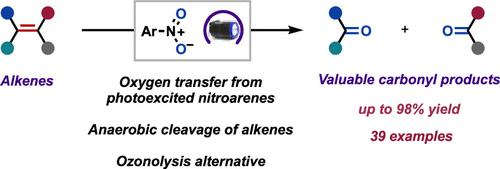
Herein we report the anaerobic cleavage of alkenes into carbonyl compounds using nitroarenes as oxygen transfer reagents under visible light. This approach serves as a safe and practical alternative to mainstream oxidative cleavage protocols, such as ozonolysis and the Lemieux–Johnson reaction. A wide range of alkenes possessing oxidatively sensitive functionalities underwent anaerobic cleavage to generate carbonyl derivatives with high efficiency and regioselectivity. Mechanistic studies support that the transformation occurs via direct photoexcitation of the nitroarene followed by a nonstereospecific radical cycloaddition event with alkenes. This leads to 1,3,2- and 1,4,2-dioxazolidine intermediates that fragment to give the carbonyl products. A combination of radical clock experiments and in situ photoNMR spectroscopy revealed the identities of the key radical species and the putative aryl dioxazolidine intermediates, respectively.
中文翻译:

使用硝基芳烃进行烯烃厌氧裂解的光诱导氧转移
在这里,我们报告了在可见光下使用硝基芳烃作为氧转移试剂将烯烃厌氧裂解成羰基化合物。这种方法可作为主流氧化裂解方案(如臭氧分解和 Lemieux-Johnson 反应)的安全且实用的替代方案。具有氧化敏感性官能团的多种烯烃经历厌氧裂解以产生具有高效率和区域选择性的羰基衍生物。机理研究支持通过硝基芳烃的直接光激发以及随后与烯烃的非立体特异性自由基环加成反应发生转化。这导致 1,3,2- 和 1,4,2-二恶唑烷中间体断裂以产生羰基产物。激进时钟实验和原位实验的结合光核磁共振光谱分别揭示了关键自由基物种和推定的芳基二恶唑烷中间体的身份。
更新日期:2022-08-05
中文翻译:

使用硝基芳烃进行烯烃厌氧裂解的光诱导氧转移
在这里,我们报告了在可见光下使用硝基芳烃作为氧转移试剂将烯烃厌氧裂解成羰基化合物。这种方法可作为主流氧化裂解方案(如臭氧分解和 Lemieux-Johnson 反应)的安全且实用的替代方案。具有氧化敏感性官能团的多种烯烃经历厌氧裂解以产生具有高效率和区域选择性的羰基衍生物。机理研究支持通过硝基芳烃的直接光激发以及随后与烯烃的非立体特异性自由基环加成反应发生转化。这导致 1,3,2- 和 1,4,2-二恶唑烷中间体断裂以产生羰基产物。激进时钟实验和原位实验的结合光核磁共振光谱分别揭示了关键自由基物种和推定的芳基二恶唑烷中间体的身份。



























 京公网安备 11010802027423号
京公网安备 11010802027423号