当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Direct Benzylic C(sp3)–O Coupling with Alcohol via Site-Selective C(sp3)–H Cleavage at Room Temperature through a Remote Directing Group-Enabled Radical Relay Strategy
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2022-08-05 , DOI: 10.1021/acs.joc.2c00884 Fang Wang 1 , Jiaming Chen 1 , Xiaoqi Jia 1 , Dailin Zhuang 1 , Zhenyang Wan 1 , Lifang Ma 1 , Ziyuan Li 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2022-08-05 , DOI: 10.1021/acs.joc.2c00884 Fang Wang 1 , Jiaming Chen 1 , Xiaoqi Jia 1 , Dailin Zhuang 1 , Zhenyang Wan 1 , Lifang Ma 1 , Ziyuan Li 1
Affiliation
|
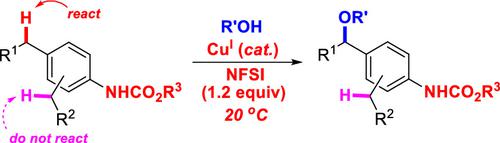
Employing a low loading of the terminal oxidant, a remote directing group-enabled radical relay strategy for benzylic direct C(sp3)–H alkoxylation with alcohols at room temperature is developed. Satisfactory site-selectivity, chemoselectivity, and reaction scope are achieved under simple and mild conditions, and no ligand or additive is required. Mechanistic studies, ready conversions of the directing group, and other benzylic functionalizations currently under development in our laboratory further indicate the promising potentials of this remote directing group-enabled radical relay strategy.
中文翻译:

直接苄基 C(sp3)–O 通过位点选择性 C(sp3)–H 裂解在室温下通过远程引导基团启用自由基中继策略与醇偶联
采用低负载的末端氧化剂,开发了一种在室温下与醇直接进行苄基直接 C(sp 3 )-H 烷氧基化的远程导向基团自由基中继策略。在简单温和的条件下实现了令人满意的位点选择性、化学选择性和反应范围,无需配体或添加剂。我们实验室目前正在开发的机械研究、导向基团的现成转换和其他苄基官能化进一步表明了这种远程导向基团激活的自由基中继策略的潜力。
更新日期:2022-08-05
中文翻译:

直接苄基 C(sp3)–O 通过位点选择性 C(sp3)–H 裂解在室温下通过远程引导基团启用自由基中继策略与醇偶联
采用低负载的末端氧化剂,开发了一种在室温下与醇直接进行苄基直接 C(sp 3 )-H 烷氧基化的远程导向基团自由基中继策略。在简单温和的条件下实现了令人满意的位点选择性、化学选择性和反应范围,无需配体或添加剂。我们实验室目前正在开发的机械研究、导向基团的现成转换和其他苄基官能化进一步表明了这种远程导向基团激活的自由基中继策略的潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号