European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2022-08-05 , DOI: 10.1016/j.ejmech.2022.114629 Rehab H Abd El-Aleam 1 , Ahmed M Sayed 2 , Mostafa N Taha 3 , Riham F George 4 , Hanan H Georgey 5 , Hamdy M Abdel-Rahman 6
|
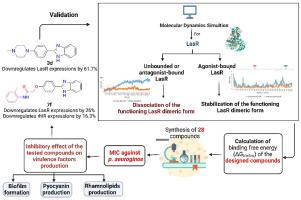
Quorum sensing (QS) inhibition is one of the potential methods to target bacterial infection. In this study, comprehensive molecular dynamics simulation (MDS) experiments were conducted on the LasR structure to understand its structural dynamic behavior either in its ligand-free form or in its ligand-bound form (i.e. agonist or antagonist). The results revealed that LasR structure is significantly unstable in its ligand-free and antagonist-bound forms and such structural instability led eventually to complete dissociation of the functioning LasR dimeric form. Accordingly, twenty-eight benzimidazole derivatives were designed, synthesized as potential LasR antagonists, and characterized in vitro as QS inhibitors. Compounds 3d and 7f disclosed the highest percentage inhibition in biofilm formation, pyocyanin, and rhamnolipids production in Pseudomonas aeruginosa (71.70%, 68.70%, 54.00%) and (68.90%, 68.00%, 51.80%), respectively. MDS experiments revealed that these compounds as inhibitors, particularly, 3d, 7f, 8a, and 9g induce LasR structure instability and complete dissociation of its functioning dimeric form similarly to the previously reported inhibitor bromophenethyl-2-nitrobenzamide (BPNB). Furthermore, gene expression assays as another mechanism targeting quorum sensing genes to prove the inhibitory activity of these compounds on virulence factors, revealed that a number of the synthesized compounds were able to downregulate lasR (e.g. 3d and 7f by 61.70% and 26.00%, respectively) and rhlR (e.g. 7f by 16.30%) expressions. The results presented here provide a functional model for LasR that could guide future design of LasR inhibitors.
中文翻译:

新型苯并咪唑衍生物作为抑制 LasR 的潜在铜绿假单胞菌抗生物膜剂的设计和合成:来自综合分子动力学模拟和体外研究的证据
群体感应 (QS) 抑制是针对细菌感染的潜在方法之一。在这项研究中,对 LasR 结构进行了全面的分子动力学模拟 (MDS) 实验,以了解其无配体形式或配体结合形式(即激动剂或拮抗剂)的结构动力学行为。结果表明,LasR 结构在其无配体和拮抗剂结合形式中显着不稳定,这种结构不稳定性最终导致功能性 LasR 二聚体形式的完全解离。因此,设计、合成了 28 种苯并咪唑衍生物作为潜在的 LasR 拮抗剂,并在体外作为 QS 抑制剂进行了表征。化合物3d和7f分别揭示了铜绿假单胞菌(71.70%、68.70%、54.00%)和(68.90%、68.00%、51.80%)中生物膜形成、绿脓素和鼠李糖脂产生的最高抑制百分比。MDS 实验表明,这些化合物作为抑制剂,特别是3d、7f、8a和9g ,会诱导 LasR 结构的不稳定性和其功能二聚体形式的完全解离,类似于先前报道的抑制剂溴苯乙基-2-硝基苯甲酰胺 (BPNB)。此外,基因表达测定作为另一种针对群体感应基因的机制,以证明这些化合物对毒力因子的抑制活性,表明许多合成的化合物能够下调lasR(例如3d和7f分别为 61.70% 和 26.00%)和rhlR(例如7f为 16.30%)表达。这里展示的结果为 LasR 提供了一个功能模型,可以指导未来 LasR 抑制剂的设计。



























 京公网安备 11010802027423号
京公网安备 11010802027423号