当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Atroposelective Total Synthesis of Darobactin A
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2022-08-04 , DOI: 10.1021/jacs.2c05892 You-Chen Lin 1 , Fabian Schneider 1 , Kelly J Eberle 1 , Debora Chiodi 1 , Hugh Nakamura 1 , Solomon H Reisberg 1 , Jason Chen 1 , Masato Saito 1 , Phil S Baran 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2022-08-04 , DOI: 10.1021/jacs.2c05892 You-Chen Lin 1 , Fabian Schneider 1 , Kelly J Eberle 1 , Debora Chiodi 1 , Hugh Nakamura 1 , Solomon H Reisberg 1 , Jason Chen 1 , Masato Saito 1 , Phil S Baran 1
Affiliation
|
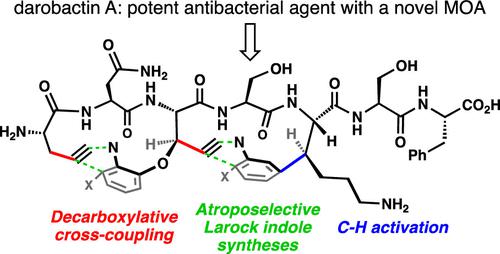
A concise, modular synthesis of the novel antibiotic darobactin A is disclosed. The synthesis successfully forges the hallmark strained macrocyclic ring systems in a sequential fashion. Key transformations include two atroposelective Larock-based macrocyclizations, one of which proceeds with exquisite regioselectivity despite bearing an unprotected alkyne. The synthesis is designed with medicinal chemistry considerations in mind, appending key portions of the molecule at a late stage. Requisite unnatural amino acid building blocks are easily prepared in an enantiopure form using C–H activation and decarboxylative cross-coupling tactics.
中文翻译:

Darobactin A 的肌细胞选择性全合成
公开了新型抗生素darobactin A的简明、模块化合成。该合成以连续方式成功地形成了标志性的应变大环系统。关键的转化包括两种基于 Larock 的天体选择性大环化,其中一种尽管带有未受保护的炔烃,但仍然具有精致的区域选择性。合成的设计考虑了药物化学因素,在后期添加分子的关键部分。使用 C-H 活化和脱羧交叉偶联策略可以轻松制备对映体纯形式的必要非天然氨基酸构建块。
更新日期:2022-08-04
中文翻译:

Darobactin A 的肌细胞选择性全合成
公开了新型抗生素darobactin A的简明、模块化合成。该合成以连续方式成功地形成了标志性的应变大环系统。关键的转化包括两种基于 Larock 的天体选择性大环化,其中一种尽管带有未受保护的炔烃,但仍然具有精致的区域选择性。合成的设计考虑了药物化学因素,在后期添加分子的关键部分。使用 C-H 活化和脱羧交叉偶联策略可以轻松制备对映体纯形式的必要非天然氨基酸构建块。


























 京公网安备 11010802027423号
京公网安备 11010802027423号