当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic Enantioselective α-Alkylation of Amides by Unactivated Alkyl Electrophiles
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2022-08-04 , DOI: 10.1021/jacs.2c06154 Xiaoyu Tong 1 , Felix Schneck 1 , Gregory C Fu 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2022-08-04 , DOI: 10.1021/jacs.2c06154 Xiaoyu Tong 1 , Felix Schneck 1 , Gregory C Fu 1
Affiliation
|
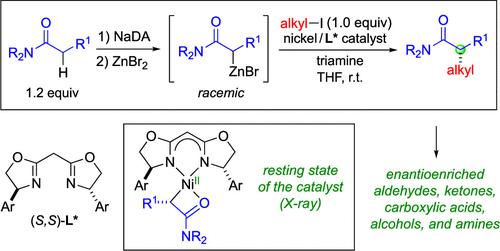
Carbonyl groups that bear an α stereocenter are commonly found in bioactive compounds, and intense effort has therefore been dedicated to the pursuit of stereoselective methods for constructing this motif. While the chiral auxiliary-enabled coupling of enolates with alkyl electrophiles represented groundbreaking progress in addressing this challenge, the next advance in the evolution of this enolate–alkylation approach would be to use a chiral catalyst to control stereochemistry. Herein we describe the achievement of this objective, demonstrating that a nickel catalyst can accomplish enantioselective intermolecular alkylations of racemic Reformatsky reagents with unactivated electrophiles; the resulting α-alkylated carbonyl compounds can be converted in one additional step into a diverse array of ubiquitous families of chiral molecules. Applying a broad spectrum of mechanistic tools, we have gained insight into key intermediates (including the alkylnickel(II) resting state) and elementary steps of the catalytic cycle.
中文翻译:

未活化烷基亲电子试剂对酰胺的催化对映选择性 α-烷基化
带有α立构中心的羰基常见于生物活性化合物中,因此人们一直致力于寻找构建该基序的立体选择性方法。虽然烯醇化物与烷基亲电子试剂的手性辅助偶联代表了解决这一挑战的突破性进展,但这种烯醇化物烷基化方法的下一个进展将是使用手性催化剂来控制立体化学。在此,我们描述了这一目标的实现,证明镍催化剂可以实现外消旋Reformatsky试剂与未活化的亲电子试剂的对映选择性分子间烷基化;所得到的α-烷基化羰基化合物可以通过一个额外的步骤转化为一系列广泛存在的手性分子家族。通过应用广泛的机械工具,我们深入了解了催化循环的关键中间体(包括烷基镍 (II) 静息态)和基本步骤。
更新日期:2022-08-04
中文翻译:

未活化烷基亲电子试剂对酰胺的催化对映选择性 α-烷基化
带有α立构中心的羰基常见于生物活性化合物中,因此人们一直致力于寻找构建该基序的立体选择性方法。虽然烯醇化物与烷基亲电子试剂的手性辅助偶联代表了解决这一挑战的突破性进展,但这种烯醇化物烷基化方法的下一个进展将是使用手性催化剂来控制立体化学。在此,我们描述了这一目标的实现,证明镍催化剂可以实现外消旋Reformatsky试剂与未活化的亲电子试剂的对映选择性分子间烷基化;所得到的α-烷基化羰基化合物可以通过一个额外的步骤转化为一系列广泛存在的手性分子家族。通过应用广泛的机械工具,我们深入了解了催化循环的关键中间体(包括烷基镍 (II) 静息态)和基本步骤。


























 京公网安备 11010802027423号
京公网安备 11010802027423号