当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solubility Determination and Thermodynamic Modeling of Methanesulfonamide in 13 Pure Solvents at Temperatures of 283.15–323.15 K
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2022-08-04 , DOI: 10.1021/acs.jced.2c00012 Xiaoli Wu 1 , Shui Wang 1 , Xinyi Lü 1 , Yixin Qu 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2022-08-04 , DOI: 10.1021/acs.jced.2c00012 Xiaoli Wu 1 , Shui Wang 1 , Xinyi Lü 1 , Yixin Qu 1
Affiliation
|
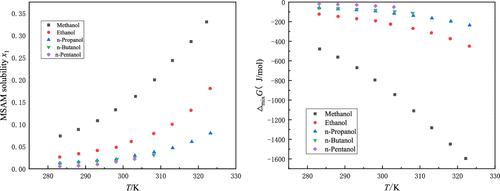
In this paper, the solubility of methanesulfonamide (MSAM) in selected 13 pure solvents including 9 alcohols (methanol, ethanol, n-propanol, iso-propanol, n-butanol, sec-butanol, iso-butanol, n-pentanol, and iso-pentanol) and 4 esters (ethyl acetate, propyl acetate, iso-propyl acetate, and methyl propionate) at atmospheric pressure and temperatures from 283.15 to 323.15 K was determined. The solubility as a function of temperature was regressed using modified Apelblat, Van’t Hoff, λh, Wilson, and nonrandom two-liquid (NRTL) models. Based on the experimental and the simulation results, the thermodynamic mixing properties of the solutions, mixing Gibbs energy, enthalpy, and entropy were evaluated.
中文翻译:

甲磺酰胺在 283.15–323.15 K 温度下在 13 种纯溶剂中的溶解度测定和热力学模型
本文研究了甲磺酰胺(MSAM)在选定的 13 种纯溶剂中的溶解度,包括 9 种醇(甲醇、乙醇、正丙醇、异丙醇、正丁醇、仲丁醇、异丁醇、正戊醇和异丙醇)在大气压和 283.15 至 323.15 K 的温度下测定了 4 种酯(乙酸乙酯、乙酸丙酯、乙酸异丙酯和丙酸甲酯)。使用改进的 Apelblat、Van't Hoff、λ h、Wilson 和非随机二液体 (NRTL) 模型对作为温度函数的溶解度进行回归。根据实验和模拟结果,对溶液的热力学混合特性、混合吉布斯能量、焓和熵进行了评价。
更新日期:2022-08-04
中文翻译:

甲磺酰胺在 283.15–323.15 K 温度下在 13 种纯溶剂中的溶解度测定和热力学模型
本文研究了甲磺酰胺(MSAM)在选定的 13 种纯溶剂中的溶解度,包括 9 种醇(甲醇、乙醇、正丙醇、异丙醇、正丁醇、仲丁醇、异丁醇、正戊醇和异丙醇)在大气压和 283.15 至 323.15 K 的温度下测定了 4 种酯(乙酸乙酯、乙酸丙酯、乙酸异丙酯和丙酸甲酯)。使用改进的 Apelblat、Van't Hoff、λ h、Wilson 和非随机二液体 (NRTL) 模型对作为温度函数的溶解度进行回归。根据实验和模拟结果,对溶液的热力学混合特性、混合吉布斯能量、焓和熵进行了评价。

























 京公网安备 11010802027423号
京公网安备 11010802027423号