当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chemoselective Oxidation of Thiols with Oxoammonium Cations
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2022-08-04 , DOI: 10.1021/acs.joc.2c01097 Shayne M Weierbach 1 , Robert P Reynolds 1 , Shannon M Stephens 1 , Kostantinos V Vlasakakis 1 , Ramsey T Ritter 1 , Olivia M White 1 , Nishi H Patel 1 , Eric C Hayes 1 , Sydney Dunmire 1 , Kyle M Lambert 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2022-08-04 , DOI: 10.1021/acs.joc.2c01097 Shayne M Weierbach 1 , Robert P Reynolds 1 , Shannon M Stephens 1 , Kostantinos V Vlasakakis 1 , Ramsey T Ritter 1 , Olivia M White 1 , Nishi H Patel 1 , Eric C Hayes 1 , Sydney Dunmire 1 , Kyle M Lambert 1
Affiliation
|
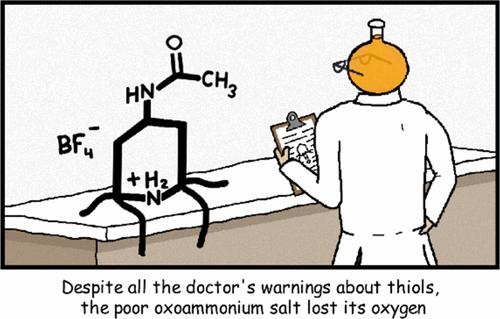
The oxidation of various aryl and aliphatic thiols with the commercially available and environmentally benign reagent Bobbitt’s salt (1) has been investigated. The reaction affords the corresponding disulfide products in good to excellent yields (71–99%) and can be accomplished in water, methanol, or acetonitrile solvent. Moreover, the process is highly chemoselective, tolerating traditionally oxidation-labile groups such as free amines and alcohols. Combined experimental and computational studies reveal that the oxidation takes place via a polar two-electron process with concomitant and unexpected deoxygenation of the oxoammonium cation through homolysis of the weak N–O bond, differing from prototypical radical-based thiol couplings. This unusual consumption of the oxidant has significant implications for the development of new nitroxide-based radical traps for probing S-centered radicals, the advancement of new electrochemical or catalytic processes involving nitroxide/oxoammonium salt redox couples, and applications to biological systems.
中文翻译:

硫醇与氧铵阳离子的化学选择性氧化
已经研究了使用市售且环境友好的试剂博比特盐( 1 )氧化各种芳基和脂肪族硫醇。该反应以良好至优异的收率(71-99%)提供相应的二硫化物产物,并且可以在水、甲醇或乙腈溶剂中完成。此外,该过程具有高度化学选择性,可耐受传统的氧化不稳定基团,例如游离胺和醇。实验和计算相结合的研究表明,氧化是通过极性双电子过程发生的,同时通过弱 N-O 键的均裂,伴随着氧铵阳离子的意外脱氧,这与典型的基于自由基的硫醇偶联不同。这种氧化剂的异常消耗对于开发用于探测S中心自由基的新型氮氧自由基陷阱、涉及氮氧自由基/氧铵盐氧化还原对的新电化学或催化过程以及在生物系统中的应用具有重要意义。
更新日期:2022-08-04
中文翻译:

硫醇与氧铵阳离子的化学选择性氧化
已经研究了使用市售且环境友好的试剂博比特盐( 1 )氧化各种芳基和脂肪族硫醇。该反应以良好至优异的收率(71-99%)提供相应的二硫化物产物,并且可以在水、甲醇或乙腈溶剂中完成。此外,该过程具有高度化学选择性,可耐受传统的氧化不稳定基团,例如游离胺和醇。实验和计算相结合的研究表明,氧化是通过极性双电子过程发生的,同时通过弱 N-O 键的均裂,伴随着氧铵阳离子的意外脱氧,这与典型的基于自由基的硫醇偶联不同。这种氧化剂的异常消耗对于开发用于探测S中心自由基的新型氮氧自由基陷阱、涉及氮氧自由基/氧铵盐氧化还原对的新电化学或催化过程以及在生物系统中的应用具有重要意义。



























 京公网安备 11010802027423号
京公网安备 11010802027423号