Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solubilization of Aldehydes and Amines in Aqueous CiEj Surfactant Aggregates: Solubilization Capacity and Aggregate Properties
Langmuir ( IF 3.9 ) Pub Date : 2022-08-04 , DOI: 10.1021/acs.langmuir.2c01463 Peter Kroll 1 , Gabriele Sadowski 1 , Christoph Brandenbusch 1
Langmuir ( IF 3.9 ) Pub Date : 2022-08-04 , DOI: 10.1021/acs.langmuir.2c01463 Peter Kroll 1 , Gabriele Sadowski 1 , Christoph Brandenbusch 1
Affiliation
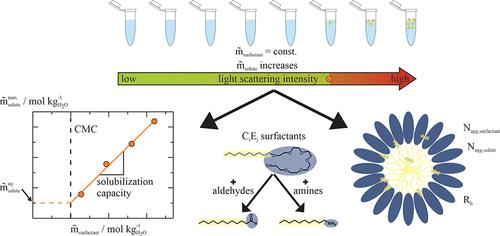
|
Hydroformylation of olefins to aldehydes and subsequent reductive amination of aldehydes to amines takes place in an aqueous system using a water-soluble catalyst. It is limited to short-chain molecules due to an insufficient solubility of long-chain molecules in water. A promising approach to increase the solubility of long-chain aldehydes and amines is the addition of surfactants to the aqueous phase. In this work, we thus determined the solubilization capacity (SC) of different nonionic CiEj surfactants (C8E6, C10E6, and C10E8) toward long-chain aldehydes and amines. We used static and dynamic light scattering techniques to investigate the influence of both the surfactant and solute molecular structures on the SC as well as on the aggregation number (Nagg) and hydrodynamic radius (Rh) of mixed aggregates. Our data reveals that an optimum ratio of hydrophobic to hydrophilic chain length of CiEj surfactants exists where the SC toward long-chain aldehydes and amines possesses a maximum. Further, the size of the aggregates (Nagg, Rh) passes through a minimum upon amine solubilization, while upon aldehyde solubilization, the aggregate size increases gradually. The results shown in this work give valuable insights to the solubilization of aldehydes and n-amines into nonionic CiEj surfactants and facilitate the search of suitable surfactants for hydroformylation and reductive amination as “green” solvents based on the detailed knowledge about the aggregate structure.
中文翻译:

醛和胺在含水 CiEj 表面活性剂聚集体中的增溶:增溶能力和聚集体性质
烯烃加氢甲酰化为醛,随后醛还原胺化为胺,使用水溶性催化剂在水性体系中进行。由于长链分子在水中的溶解度不足,因此仅限于短链分子。增加长链醛和胺溶解度的一种有前途的方法是在水相中添加表面活性剂。因此,在这项工作中,我们确定了不同非离子 C i E j表面活性剂(C 8 E 6、C 10 E 6和 C 10 E 8 )的增溶能力 (SC)) 对长链醛和胺。我们使用静态和动态光散射技术来研究表面活性剂和溶质分子结构对 SC 以及混合聚集体的聚集数 ( N agg ) 和流体动力学半径 ( R h ) 的影响。我们的数据表明,C i E j表面活性剂的疏水链长度与亲水链长度的最佳比率存在于其中 SC 对长链醛和胺具有最大值的地方。此外,聚集体的大小 ( N agg , R h) 在胺溶解时通过最小值,而在醛溶解时,聚集体尺寸逐渐增加。这项工作中显示的结果为醛类和正胺在非离子 C i E j表面活性剂中的增溶提供了有价值的见解,并有助于基于对聚集体的详细了解,寻找合适的用于加氢甲酰化和还原胺化的表面活性剂作为“绿色”溶剂结构体。
更新日期:2022-08-04
中文翻译:

醛和胺在含水 CiEj 表面活性剂聚集体中的增溶:增溶能力和聚集体性质
烯烃加氢甲酰化为醛,随后醛还原胺化为胺,使用水溶性催化剂在水性体系中进行。由于长链分子在水中的溶解度不足,因此仅限于短链分子。增加长链醛和胺溶解度的一种有前途的方法是在水相中添加表面活性剂。因此,在这项工作中,我们确定了不同非离子 C i E j表面活性剂(C 8 E 6、C 10 E 6和 C 10 E 8 )的增溶能力 (SC)) 对长链醛和胺。我们使用静态和动态光散射技术来研究表面活性剂和溶质分子结构对 SC 以及混合聚集体的聚集数 ( N agg ) 和流体动力学半径 ( R h ) 的影响。我们的数据表明,C i E j表面活性剂的疏水链长度与亲水链长度的最佳比率存在于其中 SC 对长链醛和胺具有最大值的地方。此外,聚集体的大小 ( N agg , R h) 在胺溶解时通过最小值,而在醛溶解时,聚集体尺寸逐渐增加。这项工作中显示的结果为醛类和正胺在非离子 C i E j表面活性剂中的增溶提供了有价值的见解,并有助于基于对聚集体的详细了解,寻找合适的用于加氢甲酰化和还原胺化的表面活性剂作为“绿色”溶剂结构体。


























 京公网安备 11010802027423号
京公网安备 11010802027423号