当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solid–Liquid Phase Equilibria and Boron Species Distribution in the Quaternary System LiCl–LiBO2–Li2B4O7–H2O at T = 323.15 K and P = 0.1 MPa
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2022-08-04 , DOI: 10.1021/acs.jced.2c00261 Tao Zhang 1 , Liwei Zhuang 1 , Dan Li 1 , Lingzong Meng 1 , Tianlong Deng 2 , Yafei Guo 2 , Yong Ma 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2022-08-04 , DOI: 10.1021/acs.jced.2c00261 Tao Zhang 1 , Liwei Zhuang 1 , Dan Li 1 , Lingzong Meng 1 , Tianlong Deng 2 , Yafei Guo 2 , Yong Ma 1
Affiliation
|
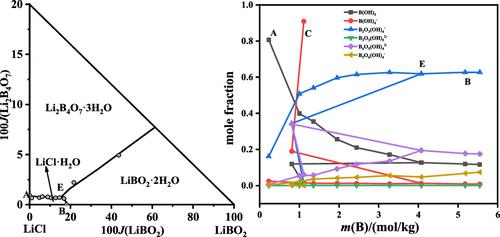
The solubilities of salts, refractive indices, and pH values of the equilibrium liquid phases in the quaternary system LiCl–LiBO2–Li2B4O7–H2O at 323.15 K were investigated by the isothermal dissolution method for the first time. The dry-salt diagram of the quaternary system consisted of one invariant point, three univariant curves and three crystallization fields corresponding to LiCl·H2O, LiBO2·2H2O, and Li2B4O7·3H2O. The solution refractive indices and pH values change regularly with the changing of concentration. Combining the equilibrium constants for different boron species, the distributions of six boron species in the mixed solution in the systems LiCl–LiBO2–H2O, LiBO2–Li2B4O7–H2O, and LiCl–Li2B4O7–LiBO2–H2O were calculated with the total boron concentration and pH values. The main boron species in the mixed solution of the quaternary system are B3O3(OH)4–, B4O5(OH)42–, B(OH)3, and B5O6(OH)4–. The boron species distributions are mainly affected by the pH values and the concentration of total boron and LiCl in the solution. The results of phase diagram and boron species distribution in the quaternary system can supply foundation data for separating lithium borate from brines.
中文翻译:

T = 323.15 K 和 P = 0.1 MPa 时第四纪体系 LiCl–LiBO2–Li2B4O7–H2O 中的固液相平衡和硼种类分布
首次采用等温溶解法研究了323.15 K时四元体系LiCl-LiBO 2 -Li 2 B 4 O 7 -H 2 O中盐的溶解度、折射率和平衡液相的pH值。四元体系的干盐图由一个不变量点、三个单变量曲线和三个结晶场组成,分别对应于LiCl·H 2 O、LiBO 2 ·2H 2 O、Li 2 B 4 O 7 ·3H 2O.溶液的折射率和pH值随着浓度的变化而规律地变化。结合不同硼物种的平衡常数,得到LiCl-LiBO 2 -H 2 O、LiBO 2 -Li 2 B 4 O 7 -H 2 O和LiCl-Li 2体系混合溶液中六种硼物种的分布B 4 O 7 -LiBO 2 -H 2 O 用总硼浓度和pH值计算。四元体系混合溶液中的主要硼种类为 B 3 O 3 (OH) 4 –, B 4 O 5 (OH) 4 2– , B(OH) 3 , 和 B 5 O 6 (OH) 4 –。硼种类分布主要受溶液中 pH 值和总硼和 LiCl 浓度的影响。四元体系中的相图和硼物种分布结果可为硼酸锂与卤水的分离提供基础数据。
更新日期:2022-08-04
中文翻译:

T = 323.15 K 和 P = 0.1 MPa 时第四纪体系 LiCl–LiBO2–Li2B4O7–H2O 中的固液相平衡和硼种类分布
首次采用等温溶解法研究了323.15 K时四元体系LiCl-LiBO 2 -Li 2 B 4 O 7 -H 2 O中盐的溶解度、折射率和平衡液相的pH值。四元体系的干盐图由一个不变量点、三个单变量曲线和三个结晶场组成,分别对应于LiCl·H 2 O、LiBO 2 ·2H 2 O、Li 2 B 4 O 7 ·3H 2O.溶液的折射率和pH值随着浓度的变化而规律地变化。结合不同硼物种的平衡常数,得到LiCl-LiBO 2 -H 2 O、LiBO 2 -Li 2 B 4 O 7 -H 2 O和LiCl-Li 2体系混合溶液中六种硼物种的分布B 4 O 7 -LiBO 2 -H 2 O 用总硼浓度和pH值计算。四元体系混合溶液中的主要硼种类为 B 3 O 3 (OH) 4 –, B 4 O 5 (OH) 4 2– , B(OH) 3 , 和 B 5 O 6 (OH) 4 –。硼种类分布主要受溶液中 pH 值和总硼和 LiCl 浓度的影响。四元体系中的相图和硼物种分布结果可为硼酸锂与卤水的分离提供基础数据。


























 京公网安备 11010802027423号
京公网安备 11010802027423号