当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Intrinsic and Extrinsic Limitations to the Design and Optimization of Inhibitors of Lipid Peroxidation and Associated Cell Death
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2022-08-03 , DOI: 10.1021/jacs.2c05252 Luke A Farmer 1 , Zijun Wu 1 , Jia-Fei Poon 1 , Omkar Zilka 1 , Svenja M Lorenz 2 , Stephanie Huehn 2 , Bettina Proneth 2 , Marcus Conrad 2 , Derek A Pratt 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2022-08-03 , DOI: 10.1021/jacs.2c05252 Luke A Farmer 1 , Zijun Wu 1 , Jia-Fei Poon 1 , Omkar Zilka 1 , Svenja M Lorenz 2 , Stephanie Huehn 2 , Bettina Proneth 2 , Marcus Conrad 2 , Derek A Pratt 1
Affiliation
|
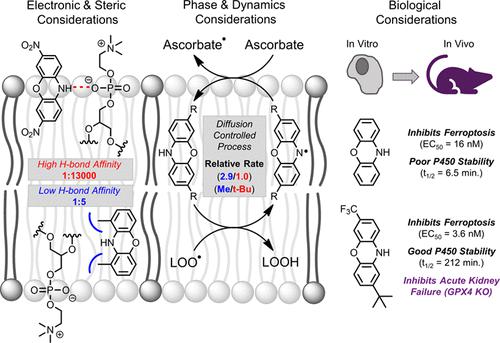
The archetype inhibitors of ferroptosis, ferrostatin-1 and liproxstatin-1, were identified via high-throughput screening of compound libraries for cytoprotective activity. These compounds have been shown to inhibit ferroptosis by suppressing propagation of lipid peroxidation, the radical chain reaction that drives cell death. Herein, we present the first rational design and optimization of ferroptosis inhibitors targeting this mechanism of action. Engaging the most potent radical-trapping antioxidant (RTA) scaffold known (phenoxazine, PNX), and its less reactive chalcogen cousin (phenothiazine, PTZ), we explored structure–reactivity–potency relationships to elucidate the intrinsic and extrinsic limitations of this approach. The results delineate the roles of inherent RTA activity, H-bonding interactions with phospholipid headgroups, and lipid solubility in determining activity/potency. We show that modifications which increase inherent RTA activity beyond that of the parent compounds do not substantially improve RTA kinetics in phospholipids or potency in cells, while modifications that decrease intrinsic RTA activity lead to corresponding erosions to both. The apparent “plateau” of RTA activity in phospholipid bilayers (kinh ∼ 2 × 105 M–1 s–1) and cell potency (EC50 ∼ 4 nM) may be the result of diffusion-controlled reactivity between the RTA and lipid-peroxyl radicals and/or the potential limitations on RTA turnover/regeneration by endogenous reductants. The metabolic stability of selected derivatives was assessed to identify a candidate for in vivo experimentation as a proof-of-concept. This PNX-derivative demonstrated stability in mouse liver microsomes comparable to liproxstatin-1 and was successfully used to suppress acute renal failure in mice brought on by tissue-specific inactivation of the ferroptosis regulator GPX4.
中文翻译:

脂质过氧化和相关细胞死亡抑制剂设计和优化的内在和外在限制
通过对化合物库的细胞保护活性进行高通量筛选,鉴定了铁死亡的原型抑制剂 ferrostatin-1 和 liproxstatin-1。这些化合物已被证明通过抑制脂质过氧化的传播来抑制铁死亡,脂质过氧化是驱动细胞死亡的自由基链式反应。在这里,我们提出了针对这种作用机制的铁死亡抑制剂的第一个合理设计和优化。利用已知的最有效的自由基捕获抗氧化剂 (RTA) 支架(吩恶嗪,PNX)及其反应性较低的硫属元素表亲(吩噻嗪,PTZ),我们探索了结构-反应性-效价关系,以阐明这种方法的内在和外在限制。结果描述了固有 RTA 活性、与磷脂头基的 H 键相互作用、和确定活性/效力中的脂溶性。我们表明,增加固有 RTA 活性超过母体化合物的修饰不会显着改善磷脂中的 RTA 动力学或细胞中的效力,而降低固有 RTA 活性的修饰会导致两者的相应侵蚀。磷脂双分子层中 RTA 活性的明显“平台期”(k inh ∼ 2 × 10 5 M –1 s –1 ) 和细胞效力 (EC 50 ∼ 4 nM) 可能是 RTA 和脂质过氧自由基之间的扩散控制反应性和/或 RTA 转换的潜在限制的结果/通过内源性还原剂再生。评估选定衍生物的代谢稳定性,以确定体内实验的候选者作为概念验证。这种 PNX 衍生物在小鼠肝微粒体中表现出与 liproxstatin-1 相当的稳定性,并成功地用于抑制由组织特异性失活铁死亡调节剂 GPX4 引起的小鼠急性肾功能衰竭。
更新日期:2022-08-03
中文翻译:

脂质过氧化和相关细胞死亡抑制剂设计和优化的内在和外在限制
通过对化合物库的细胞保护活性进行高通量筛选,鉴定了铁死亡的原型抑制剂 ferrostatin-1 和 liproxstatin-1。这些化合物已被证明通过抑制脂质过氧化的传播来抑制铁死亡,脂质过氧化是驱动细胞死亡的自由基链式反应。在这里,我们提出了针对这种作用机制的铁死亡抑制剂的第一个合理设计和优化。利用已知的最有效的自由基捕获抗氧化剂 (RTA) 支架(吩恶嗪,PNX)及其反应性较低的硫属元素表亲(吩噻嗪,PTZ),我们探索了结构-反应性-效价关系,以阐明这种方法的内在和外在限制。结果描述了固有 RTA 活性、与磷脂头基的 H 键相互作用、和确定活性/效力中的脂溶性。我们表明,增加固有 RTA 活性超过母体化合物的修饰不会显着改善磷脂中的 RTA 动力学或细胞中的效力,而降低固有 RTA 活性的修饰会导致两者的相应侵蚀。磷脂双分子层中 RTA 活性的明显“平台期”(k inh ∼ 2 × 10 5 M –1 s –1 ) 和细胞效力 (EC 50 ∼ 4 nM) 可能是 RTA 和脂质过氧自由基之间的扩散控制反应性和/或 RTA 转换的潜在限制的结果/通过内源性还原剂再生。评估选定衍生物的代谢稳定性,以确定体内实验的候选者作为概念验证。这种 PNX 衍生物在小鼠肝微粒体中表现出与 liproxstatin-1 相当的稳定性,并成功地用于抑制由组织特异性失活铁死亡调节剂 GPX4 引起的小鼠急性肾功能衰竭。


























 京公网安备 11010802027423号
京公网安备 11010802027423号