当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rhodium-Catalyzed Selective C(sp2)–H Activation/Annulation of tert-Butyl Benzoyloxycarbamates with 1,3-Diynes: A One Step Access to Alkynylated Isocoumarins and Bis-Isocoumarins
Organic Letters ( IF 5.2 ) Pub Date : 2022-08-03 , DOI: 10.1021/acs.orglett.2c01901 Bedadyuti Vedvyas Pati 1, 2 , Shyam Kumar Banjare 1, 2 , Gopal Krushna Das Adhikari 1, 2 , Tanmayee Nanda 1, 2 , Ponneri C Ravikumar 1, 2
Organic Letters ( IF 5.2 ) Pub Date : 2022-08-03 , DOI: 10.1021/acs.orglett.2c01901 Bedadyuti Vedvyas Pati 1, 2 , Shyam Kumar Banjare 1, 2 , Gopal Krushna Das Adhikari 1, 2 , Tanmayee Nanda 1, 2 , Ponneri C Ravikumar 1, 2
Affiliation
|
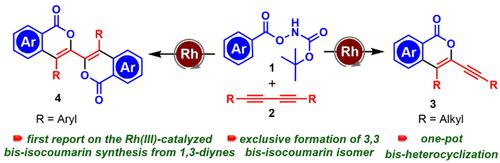
We report here a Rh(III) catalyzed regio- and stereoselective synthesis of alkynylated and bis-isocoumarin from 1,3-dialkyne. Exclusive one-pot formation of 3,3-bis-isocoumarin isomers has been achieved by eliminating several other possibilities. This is the first example of transition metal catalyzed synthesis of alkynylated and bis-isocoumarin scaffolds. The protocol is compatible with a wide range of functional groups affording good to excellent yields. Several mechanistic investigations, including deuterium labeling experiments and kinetic isotope effect studies, have been carried out.
中文翻译:

铑催化选择性 C(sp2)-H 活化/环化叔丁基苯甲酰氧基氨基甲酸酯与 1,3-二炔:一步获得炔基化异香豆素和双异香豆素
我们在此报告从 1,3-二炔烃催化的炔基化和双异香豆素的区域选择性和立体选择性合成。通过消除其他几种可能性,已经实现了 3,3-双-异香豆素异构体的独家一锅法形成。这是过渡金属催化合成炔基化和双异香豆素支架的第一个例子。该协议与广泛的功能组兼容,可提供良好的产量。已经进行了一些机械研究,包括氘标记实验和动力学同位素效应研究。
更新日期:2022-08-03
中文翻译:

铑催化选择性 C(sp2)-H 活化/环化叔丁基苯甲酰氧基氨基甲酸酯与 1,3-二炔:一步获得炔基化异香豆素和双异香豆素
我们在此报告从 1,3-二炔烃催化的炔基化和双异香豆素的区域选择性和立体选择性合成。通过消除其他几种可能性,已经实现了 3,3-双-异香豆素异构体的独家一锅法形成。这是过渡金属催化合成炔基化和双异香豆素支架的第一个例子。该协议与广泛的功能组兼容,可提供良好的产量。已经进行了一些机械研究,包括氘标记实验和动力学同位素效应研究。


























 京公网安备 11010802027423号
京公网安备 11010802027423号