Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
SLIDE: Saliva-Based SARS-CoV-2 Self-Testing with RT-LAMP in a Mobile Device
ACS Sensors ( IF 8.9 ) Pub Date : 2022-08-03 , DOI: 10.1021/acssensors.2c01023 Zifan Tang 1 , Jiarui Cui 1 , Aneesh Kshirsagar 1 , Tianyi Liu 1 , Michele Yon 2 , Suresh V Kuchipudi 2, 3 , Weihua Guan 1, 4
ACS Sensors ( IF 8.9 ) Pub Date : 2022-08-03 , DOI: 10.1021/acssensors.2c01023 Zifan Tang 1 , Jiarui Cui 1 , Aneesh Kshirsagar 1 , Tianyi Liu 1 , Michele Yon 2 , Suresh V Kuchipudi 2, 3 , Weihua Guan 1, 4
Affiliation
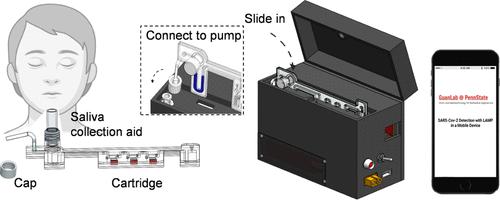
|
Regular, accurate, rapid, and inexpensive self-testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is urgently needed to quell pandemic propagation. The existing at-home nucleic acid testing (NAT) test has high sensitivity and specificity, but it requires users to mail the sample to the central lab, which often takes 3–5 days to obtain the results. On the other hand, rapid antigen tests for the SARS-CoV-2 antigen provide a fast sample to answer the test (15 min). However, the sensitivity of antigen tests is 30 to 40% lower than nucleic acid testing, which could miss a significant portion of infected patients. Here, we developed a fully integrated SARS-CoV-2 reverse transcription loop-mediated isothermal amplification (RT-LAMP) device using a self-collected saliva sample. This platform can automatically handle the complexity and can perform the functions, including (1) virus particles’ thermal lysis preparation, (2) sample dispensing, (3) target sequence RT-LAMP amplification, (4) real-time detection, and (5) result report and communication. With a turnaround time of less than 45 min, our device achieved the limit of detection (LoD) of 5 copies/μL of the saliva sample, which is comparable with the LoD (6 copies/μL) using FDA-approved quantitative real-time polymerase chain reaction (qRT-PCR) assays with the same heat-lysis saliva sample preparation method. With clinical samples, our platform showed a good agreement with the results from the gold-standard RT-PCR method. These results show that our platform can perform self-administrated SARS-CoV-2 nucleic acid testing by laypersons with noninvasive saliva samples. We believe that our self-testing platform will have an ongoing benefit for COVID-19 control and fighting future pandemics.
中文翻译:

幻灯片:在移动设备中使用 RT-LAMP 进行基于唾液的 SARS-CoV-2 自测试
迫切需要对严重急性呼吸综合征冠状病毒 2 (SARS-CoV-2) 进行定期、准确、快速和廉价的自我检测,以平息大流行的传播。现有的家庭核酸检测(NAT)检测灵敏度和特异性较高,但需要用户将样本邮寄到中心实验室,往往需要3-5天才能得到结果。另一方面,SARS-CoV-2 抗原的快速抗原测试提供了快速样本来回答测试(15 分钟)。然而,抗原检测的敏感性比核酸检测低30%至40%,这可能会漏掉很大一部分感染患者。在这里,我们使用自行收集的唾液样本开发了一种完全集成的 SARS-CoV-2 逆转录环介导等温扩增 (RT-LAMP) 装置。该平台可以自动处理复杂性并可以执行以下功能,包括(1)病毒颗粒热裂解制备,(2)样品分配,(3)目标序列RT-LAMP扩增,(4)实时检测,以及( 5)结果报告及沟通。我们的设备的周转时间不到 45 分钟,唾液样本的检测限 (LoD) 为 5 拷贝/μL,这与使用 FDA 批准的定量实时检测的 LoD (6 拷贝/μL) 相当聚合酶链反应 (qRT-PCR) 检测采用相同的热裂解唾液样品制备方法。对于临床样本,我们的平台与金标准 RT-PCR 方法的结果非常吻合。这些结果表明,我们的平台可以由外行人员使用非侵入性唾液样本进行自我管理的 SARS-CoV-2 核酸检测。我们相信,我们的自测平台将为控制 COVID-19 和对抗未来的流行病带来持续的好处。
更新日期:2022-08-03
中文翻译:

幻灯片:在移动设备中使用 RT-LAMP 进行基于唾液的 SARS-CoV-2 自测试
迫切需要对严重急性呼吸综合征冠状病毒 2 (SARS-CoV-2) 进行定期、准确、快速和廉价的自我检测,以平息大流行的传播。现有的家庭核酸检测(NAT)检测灵敏度和特异性较高,但需要用户将样本邮寄到中心实验室,往往需要3-5天才能得到结果。另一方面,SARS-CoV-2 抗原的快速抗原测试提供了快速样本来回答测试(15 分钟)。然而,抗原检测的敏感性比核酸检测低30%至40%,这可能会漏掉很大一部分感染患者。在这里,我们使用自行收集的唾液样本开发了一种完全集成的 SARS-CoV-2 逆转录环介导等温扩增 (RT-LAMP) 装置。该平台可以自动处理复杂性并可以执行以下功能,包括(1)病毒颗粒热裂解制备,(2)样品分配,(3)目标序列RT-LAMP扩增,(4)实时检测,以及( 5)结果报告及沟通。我们的设备的周转时间不到 45 分钟,唾液样本的检测限 (LoD) 为 5 拷贝/μL,这与使用 FDA 批准的定量实时检测的 LoD (6 拷贝/μL) 相当聚合酶链反应 (qRT-PCR) 检测采用相同的热裂解唾液样品制备方法。对于临床样本,我们的平台与金标准 RT-PCR 方法的结果非常吻合。这些结果表明,我们的平台可以由外行人员使用非侵入性唾液样本进行自我管理的 SARS-CoV-2 核酸检测。我们相信,我们的自测平台将为控制 COVID-19 和对抗未来的流行病带来持续的好处。


























 京公网安备 11010802027423号
京公网安备 11010802027423号