当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The mechanism for acetate formation in electrochemical CO(2) reduction on Cu: selectivity with potential, pH, and nanostructuring
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2022-08-03 , DOI: 10.1039/d2ee01485h Hendrik H. Heenen 1, 2 , Haeun Shin 3 , Georg Kastlunger 1 , Sean Overa 3 , Joseph A. Gauthier 4, 5 , Feng Jiao 3 , Karen Chan 1
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2022-08-03 , DOI: 10.1039/d2ee01485h Hendrik H. Heenen 1, 2 , Haeun Shin 3 , Georg Kastlunger 1 , Sean Overa 3 , Joseph A. Gauthier 4, 5 , Feng Jiao 3 , Karen Chan 1
Affiliation
|
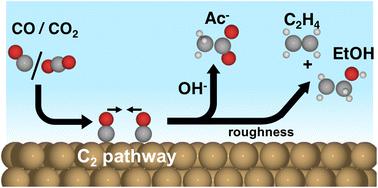
Nanostructured Cu catalysts have increased the selectivities and geometric activities for high value C–C coupled (C2) products (ethylene, ethanol, and acetate) in the electrochemical CO(2) reduction reaction (CO(2)RR). The selectivity among the high-value C2 products is also altered, where for instance the yield of acetate increases with alkalinity and is dependent on the catalyst morphology. The reaction mechanisms behind the selectivity towards acetate vs. other C2 products remain controversial. In this work, we elucidate the reaction mechanism for acetate formation by using ab initio simulations, a coupled kinetic-transport model, and loading dependent experiments. We find that trends in acetate selectivity can be rationalized from variations in electrolyte pH and the local mass transport properties of the catalyst and not from changes in Cu's intrinsic activity. The selectivity mechanism originates from the transport of ketene, a stable (closed shell) intermediate, away from the catalyst surface into solution where it reacts to form acetate. While this type of mechanism has not yet been discussed in the CO(2)RR, variants of it may explain similar selectivity fluctuations observed for other stable intermediates like CO and acetaldehyde. Our proposed mechanism suggests that acetate selectivity increases with increasing pH, decreasing catalyst roughness and significantly varies with the applied potential.
中文翻译:

Cu 电化学 CO(2) 还原中乙酸盐形成的机制:具有电位、pH 和纳米结构的选择性
纳米结构的 Cu 催化剂提高了电化学 CO (2)还原反应 (CO (2) RR)中高价值 C-C 偶联 (C 2 ) 产物(乙烯、乙醇和乙酸盐)的选择性和几何活性。高价值 C 2产物的选择性也发生了变化,例如乙酸盐的产率随着碱度的增加而增加,并且取决于催化剂的形态。对乙酸盐与其他 C 2产物的选择性背后的反应机制仍然存在争议。在这项工作中,我们从头开始阐明了乙酸盐形成的反应机理模拟、耦合动力学-运输模型和负载相关实验。我们发现醋酸盐选择性的趋势可以通过电解质 pH 值的变化和催化剂的局部传质特性来合理化,而不是通过 Cu 的内在活性的变化来合理化。选择性机制源于乙烯酮(一种稳定的(闭壳)中间体)从催化剂表面转移到溶液中,在溶液中反应形成乙酸盐。虽然这种机制尚未在 CO (2)中讨论RR,它的变体可以解释对其他稳定中间体(如 CO 和乙醛)观察到的类似选择性波动。我们提出的机制表明乙酸盐选择性随着 pH 值的增加、催化剂粗糙度的降低而增加,并且随着施加的电位而显着变化。
更新日期:2022-08-03
中文翻译:

Cu 电化学 CO(2) 还原中乙酸盐形成的机制:具有电位、pH 和纳米结构的选择性
纳米结构的 Cu 催化剂提高了电化学 CO (2)还原反应 (CO (2) RR)中高价值 C-C 偶联 (C 2 ) 产物(乙烯、乙醇和乙酸盐)的选择性和几何活性。高价值 C 2产物的选择性也发生了变化,例如乙酸盐的产率随着碱度的增加而增加,并且取决于催化剂的形态。对乙酸盐与其他 C 2产物的选择性背后的反应机制仍然存在争议。在这项工作中,我们从头开始阐明了乙酸盐形成的反应机理模拟、耦合动力学-运输模型和负载相关实验。我们发现醋酸盐选择性的趋势可以通过电解质 pH 值的变化和催化剂的局部传质特性来合理化,而不是通过 Cu 的内在活性的变化来合理化。选择性机制源于乙烯酮(一种稳定的(闭壳)中间体)从催化剂表面转移到溶液中,在溶液中反应形成乙酸盐。虽然这种机制尚未在 CO (2)中讨论RR,它的变体可以解释对其他稳定中间体(如 CO 和乙醛)观察到的类似选择性波动。我们提出的机制表明乙酸盐选择性随着 pH 值的增加、催化剂粗糙度的降低而增加,并且随着施加的电位而显着变化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号