当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
One-Pot Reductive Methylation of Nitro- and Amino-Substituted (Hetero)Aromatics with DMSO/HCOOH: Concise Synthesis of Fluorescent Dimethylamino-Functionalized Bibenzothiazole Ligands with Tunable Emission Color upon Complexation
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2022-08-02 , DOI: 10.1021/acs.joc.2c00732 Patrik Osuský 1 , Maroš Smolíček 1 , Jela Nociarová 1 , Erik Rakovský 1 , Peter Hrobárik 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2022-08-02 , DOI: 10.1021/acs.joc.2c00732 Patrik Osuský 1 , Maroš Smolíček 1 , Jela Nociarová 1 , Erik Rakovský 1 , Peter Hrobárik 1
Affiliation
|
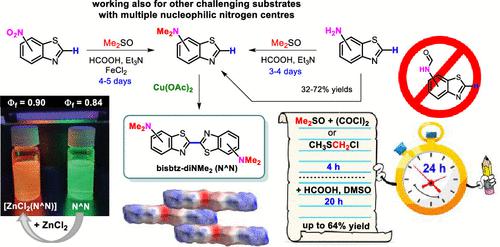
One-pot reductive N,N-dimethylation of suitable nitro- and amino-substituted (hetero)arenes can be achieved using a DMSO/HCOOH/Et3N system acting as a low-cost but efficient reducing and methylating agent. The transformation of heteroaryl-amines can be accelerated by using dimethyl sulfoxide/oxalyl chloride or chloromethyl methyl sulfide as the source of active CH3SCH2+ species, while the exclusion of HCOOH in the initial stage of the reaction allows avoiding N-formamides as resting intermediates. The developed procedures are applicable in multigram-scale synthesis, and because of the lower electrophilicity of CH3SCH2+, they also work in pathological cases, where common methylating agents provide N,N-dimethylated products in no yield or inferior yields due to concomitant side reactions. The method is particularly useful in one-pot reductive transformation of 2-H-nitrobenzazoles to corresponding N,N-dimethylamino-substituted heteroarenes. These, upon Cu(II)-catalyzed oxidative homocoupling, afford 2,2′-bibenzazoles substituted with dimethylamino groups as charge-transfer N^N ligands with intensive absorption/emission in the visible region. The fluorescence of NMe2-functionalized bibenzothiazoles remains intensive even upon complexation with ZnCl2, while emission maxima are bathochromically shifted from the green/yellow to orange/red spectral region, making these small-molecule fluorophores, exhibiting large emission quantum yields and Stokes shifts, an attractive platform for the construction of various functional dyes and light-harvesting materials with tunable emission color upon complexation.
中文翻译:

用 DMSO/HCOOH 对硝基和氨基取代的(杂)芳烃进行一锅还原甲基化:在络合时具有可调发射颜色的荧光二甲氨基官能化二苯并噻唑配体的简明合成
使用作为低成本但有效的还原剂和甲基化剂的 DMSO/HCOOH/Et 3 N 系统,可以实现合适的硝基和氨基取代的(杂)芳烃的一锅还原N,N-二甲基化。杂芳基胺的转化可以通过使用二甲亚砜/草酰氯或氯甲基甲基硫醚作为活性 CH 3 SCH 2 +物质的来源来加速,而在反应的初始阶段排除 HCOOH 可以避免N-甲酰胺作为休息中间体。由于 CH 3 SCH 2 +的亲电性较低,所开发的程序适用于多克级合成,它们也适用于病理情况,其中常见的甲基化剂提供N , N-二甲基化产物由于伴随的副反应而没有产率或低产率。该方法特别适用于将2- H-硝基苯并唑类一锅还原转化为相应的N,N-二甲基氨基-取代的杂芳烃。这些,在 Cu(II) 催化的氧化同源偶联后,提供被二甲氨基取代的 2,2'-联苯并唑作为电荷转移 N^N 配体,在可见光区域具有强烈的吸收/发射。即使在与 ZnCl 2络合后,NMe 2官能化的二苯并噻唑的荧光仍然很强烈,而发射最大值从绿色/黄色光谱区域红移到橙色/红色光谱区域,使这些小分子荧光团表现出大的发射量子产率和斯托克斯位移,成为构建各种功能染料和集光材料的有吸引力的平台在络合时具有可调的发射颜色。
更新日期:2022-08-02
中文翻译:

用 DMSO/HCOOH 对硝基和氨基取代的(杂)芳烃进行一锅还原甲基化:在络合时具有可调发射颜色的荧光二甲氨基官能化二苯并噻唑配体的简明合成
使用作为低成本但有效的还原剂和甲基化剂的 DMSO/HCOOH/Et 3 N 系统,可以实现合适的硝基和氨基取代的(杂)芳烃的一锅还原N,N-二甲基化。杂芳基胺的转化可以通过使用二甲亚砜/草酰氯或氯甲基甲基硫醚作为活性 CH 3 SCH 2 +物质的来源来加速,而在反应的初始阶段排除 HCOOH 可以避免N-甲酰胺作为休息中间体。由于 CH 3 SCH 2 +的亲电性较低,所开发的程序适用于多克级合成,它们也适用于病理情况,其中常见的甲基化剂提供N , N-二甲基化产物由于伴随的副反应而没有产率或低产率。该方法特别适用于将2- H-硝基苯并唑类一锅还原转化为相应的N,N-二甲基氨基-取代的杂芳烃。这些,在 Cu(II) 催化的氧化同源偶联后,提供被二甲氨基取代的 2,2'-联苯并唑作为电荷转移 N^N 配体,在可见光区域具有强烈的吸收/发射。即使在与 ZnCl 2络合后,NMe 2官能化的二苯并噻唑的荧光仍然很强烈,而发射最大值从绿色/黄色光谱区域红移到橙色/红色光谱区域,使这些小分子荧光团表现出大的发射量子产率和斯托克斯位移,成为构建各种功能染料和集光材料的有吸引力的平台在络合时具有可调的发射颜色。


























 京公网安备 11010802027423号
京公网安备 11010802027423号