Water Research ( IF 12.8 ) Pub Date : 2022-08-01 , DOI: 10.1016/j.watres.2022.118930 Danying Xing 1 , Shujing Shao 1 , Yuyan Yang 1 , Zuoming Zhou 1 , Guohua Jing 1 , Xiaodan Zhao 1
|
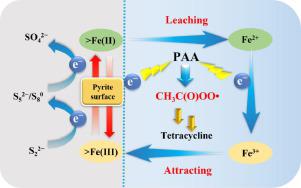
Recently, iron-based heterogenous catalysts have received much attention in the activation of peracetic acid (PAA) for generating reactive radicals to degrade organic pollutants, yet the PAA activation efficiency is compromised by the slow transformation from Fe(III) to Fe(II). Herein, considering the electron-donating ability of reducing sulfur species, a novel advanced oxidation process by combining pyrite and PAA (simplified as pyrite/PAA) for the abatement of tetracycline (TC) is proposed in this study. In the pyrite/PAA process, TC can be completely removed within 30 min under neutral conditions by the synergy of homogeneous and heterogenous Fe(II) species. CH3C(O)OO• is the main radical generated from the pyrite/PAA process responsible for TC abatement. The excellent activation properties of pyrite can be attributed to the superior electron-donating ability of reducing sulfur species to facilitate the reduction of Fe(III). Meanwhile, the complexation of leached Fe2+ with TC favors PAA activation and concomitant TC abatement. In addition, the degradation pathways of TC and the toxicity of the degradation intermediates are analyzed. The pyrite/PAA process shows an excellent TC abatement efficacy in the pH range of 4.0∼10.0. The coexistence of Cl−, HCO3−, and HPO42− exhibits negligible effect on TC abatement, while the HA slightly inhibits the abatement rate of TC. This study highlights the efficient activation of PAA by pyrite and the important role of sulfur in promoting the conversion of Fe(III) to Fe(II) in the pyrite/PAA process.
中文翻译:

黄铁矿有效活化过乙酸以减少四环素的机理研究
最近,铁基多相催化剂在活化过乙酸(PAA)以产生活性自由基以降解有机污染物方面受到了广泛关注,但由于从 Fe(III)到 Fe(II)的缓慢转变,PAA 的活化效率受到了影响。 . 在此,考虑到减少硫物质的供电子能力,本研究提出了一种通过结合黄铁矿和 PAA(简化为黄铁矿/PAA)来减少四环素(TC)的新型高级氧化工艺。在黄铁矿/PAA 工艺中,TC 可以在中性条件下通过均质和异质 Fe(II) 物种的协同作用在 30 分钟内完全去除。CH 3C(O)OO• 是黄铁矿/PAA 过程中产生的主要自由基,负责 TC 减排。黄铁矿优异的活化性能可归因于还原硫物质以促进 Fe(III) 还原的优异给电子能力。同时,浸出的 Fe 2+与 TC 的络合有利于 PAA 的活化和伴随的 TC 减少。此外,还分析了TC的降解途径和降解中间体的毒性。黄铁矿/PAA 工艺在 4.0∼10.0 的 pH 范围内显示出优异的 TC 减排效果。Cl -、HCO 3 -和HPO 4 2-的共存对 TC 减排的影响可以忽略不计,而 HA 对 TC 的减排率有轻微的抑制作用。本研究强调了黄铁矿对 PAA 的有效活化以及硫在黄铁矿/PAA 过程中促进 Fe(III) 向 Fe(II) 转化的重要作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号