Cell ( IF 64.5 ) Pub Date : 2022-08-01 , DOI: 10.1016/j.cell.2022.07.028 Shadi Tarazi 1 , Alejandro Aguilera-Castrejon 1 , Carine Joubran 1 , Nadir Ghanem 2 , Shahd Ashouokhi 1 , Francesco Roncato 1 , Emilie Wildschutz 1 , Montaser Haddad 3 , Bernardo Oldak 1 , Elidet Gomez-Cesar 1 , Nir Livnat 1 , Sergey Viukov 1 , Dmitry Lokshtanov 1 , Segev Naveh-Tassa 1 , Max Rose 1 , Suhair Hanna 4 , Calanit Raanan 5 , Ori Brenner 5 , Merav Kedmi 6 , Hadas Keren-Shaul 6 , Tsvee Lapidot 3 , Itay Maza 7 , Noa Novershtern 1 , Jacob H Hanna 1
|
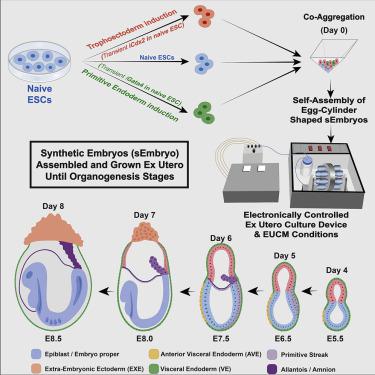
In vitro cultured stem cells with distinct developmental capacities can contribute to embryonic or extraembryonic tissues after microinjection into pre-implantation mammalian embryos. However, whether cultured stem cells can independently give rise to entire gastrulating embryo-like structures with embryonic and extraembryonic compartments remains unknown. Here, we adapt a recently established platform for prolonged ex utero growth of natural embryos to generate mouse post-gastrulation synthetic whole embryo models (sEmbryos), with both embryonic and extraembryonic compartments, starting solely from naive ESCs. This was achieved by co-aggregating non-transduced ESCs, with naive ESCs transiently expressing Cdx2 or Gata4 to promote their priming toward trophectoderm and primitive endoderm lineages, respectively. sEmbryos adequately accomplish gastrulation, advance through key developmental milestones, and develop organ progenitors within complex extraembryonic compartments similar to E8.5 stage mouse embryos. Our findings highlight the plastic potential of naive pluripotent cells to self-organize and functionally reconstitute and model the entire mammalian embryo beyond gastrulation.
中文翻译:

原肠胚形成后合成胚胎从小鼠幼稚胚胎干细胞在子宫外产生
具有独特发育能力的体外培养的干细胞在显微注射到植入前哺乳动物胚胎中后可以促进胚胎或胚胎外组织。然而,培养的干细胞是否可以独立产生具有胚胎和胚外隔室的完整原肠胚样结构仍然未知。在这里,我们调整了一个最近建立的平台,用于长时间的子宫外天然胚胎的生长以产生小鼠原肠胚形成后合成全胚胎模型 (sEmbryo),具有胚胎和胚胎外隔室,仅从原始 ESC 开始。这是通过将非转导的 ESC 与瞬时表达 Cdx2 或 Gata4 的幼稚 ESC 共同聚集来实现的,以促进它们分别启动滋养外胚层和原始内胚层谱系。sEmbryos 充分完成原肠胚形成,通过关键的发育里程碑取得进展,并在类似于 E8.5 期小鼠胚胎的复杂胚外隔室内发育器官祖细胞。我们的研究结果强调了幼稚多能细胞在原肠胚形成后进行自我组织和功能重建以及模拟整个哺乳动物胚胎的可塑性潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号