当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
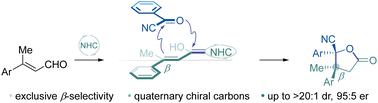
An NHC-catalyzed [3+2] cyclization of β-disubstituted enals with benzoyl cyanides
Chemical Communications ( IF 4.9 ) Pub Date : 2022-08-01 , DOI: 10.1039/d2cc04025e Wangsheng Liu 1 , Linrui Zhang 2 , Xiaoyun Liao 1 , Jiean Chen 1 , Yong Huang 3
Chemical Communications ( IF 4.9 ) Pub Date : 2022-08-01 , DOI: 10.1039/d2cc04025e Wangsheng Liu 1 , Linrui Zhang 2 , Xiaoyun Liao 1 , Jiean Chen 1 , Yong Huang 3
Affiliation
|
The NHC-catalyzed asymmetric [3+2] cyclization of benzoyl cyanides to homoenolate generated in situ from enals was reported. This methodology leads to the efficient construction of a series of chiral cyclic compounds bearing vicinal quaternary stereocenters under mild reaction conditions. Additionally, the representative large-scale and derivatization reactions of the chiral cyclic products reveal the potential synthetic utility of this protocol.
中文翻译:

NHC 催化的 β-二取代烯醛与苯甲酰氰的 [3+2] 环化
报道了 NHC 催化的苯甲酰氰化物的不对称 [3+2] 环化为由烯醛原位产生的均烯醇化物。该方法导致在温和反应条件下有效构建一系列带有邻位四元立体中心的手性环状化合物。此外,手性环状产物的代表性大规模和衍生化反应揭示了该协议的潜在合成效用。
更新日期:2022-08-04
中文翻译:

NHC 催化的 β-二取代烯醛与苯甲酰氰的 [3+2] 环化
报道了 NHC 催化的苯甲酰氰化物的不对称 [3+2] 环化为由烯醛原位产生的均烯醇化物。该方法导致在温和反应条件下有效构建一系列带有邻位四元立体中心的手性环状化合物。此外,手性环状产物的代表性大规模和衍生化反应揭示了该协议的潜在合成效用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号