Chest ( IF 9.6 ) Pub Date : 2022-07-31 , DOI: 10.1016/j.chest.2022.07.015 Hong Chen 1 , Zheng-Xu Deng 1 , Jian Sun 2 , Qiang Huang 1 , Lan Huang 1 , Yong-Hong He 1 , Chunlan Ma 2 , Ke Wang 3
|
Background
Inhaled corticosteroids (ICSs) have been used widely in the maintenance therapy of COPD. However, whether inhaled therapy containing ICSs can reduce the all-cause mortality risk and the possible benefited patient subgroups is unclear.
Research Question
Does inhaled therapy containing ICSs reduce the all-cause mortality risk in patients with COPD compared with other inhaled therapies not containing ICSs?
Study Design and Methods
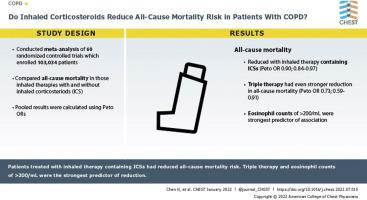
We searched PubMed, Cochrane Library, Embase, and ClinicalTrials.gov for relevant randomized clinical trials (RCTs). Pooled results were calculated using Peto ORs with corresponding 95% CIs.
Results
Sixty RCTs enrolling 103,034 patients were analyzed. Inhaled therapy containing ICSs (Peto OR, 0.90; 95% CI, 0.84-0.97), especially triple therapy (Peto OR, 0.73; 95% CI, 0.59-0.91), was associated with a reduction in the all-cause mortality risk among patients with COPD when compared with inhaled therapy without ICSs. Subgroup analyses revealed that treatment duration of > 6 months (Peto OR, 0.90; 95% CI, 0.83-0.97), medium-dose ICSs (Peto OR, 0.71; 95% CI, 0.56-0.91), low-dose ICSs (Peto OR, 0.88; 95% CI, 0.79-0.97), and budesonide (Peto OR, 0.75; 95% CI, 0.59-0.94) were involved in this association. The predictors of this association included eosinophil counts of ≥ 200/μL or percentage of ≥ 2%, documented history of ≥ 2 moderate and severe exacerbations in the previous year, Global Initiative for Chronic Obstructive Lung Disease stages III or IV, age younger than 65 years, and BMI of ≥ 25 kg/m2, among which eosinophil counts of ≥ 200/μL (Peto OR, 0.58; 95% CI, 0.36-0.95) were the strongest predictor.
Interpretation
Inhaled therapy containing ICSs, especially triple therapy, of longer than 6 months was associated with a reduction in the all-cause mortality risk in patients with COPD. The predictors of this association included medication factors and patient characteristics, among which eosinophil counts of ≥ 200/μL were the strongest predictor.
Trial Registry
PROSPERO; No.: CRD42022304725; URL: https://www.crd.york.ac.uk/prospero/
中文翻译:

吸入皮质类固醇与 COPD 患者全因死亡风险的关联
背景
吸入皮质类固醇 (ICS) 已广泛用于 COPD 的维持治疗。然而,包含 ICSs 的吸入疗法是否可以降低全因死亡风险以及可能受益的患者亚群尚不清楚。
研究问题
与不含 ICS 的其他吸入疗法相比,含有 ICS 的吸入疗法是否能降低 COPD 患者的全因死亡风险?
研究设计和方法
我们在 PubMed、Cochrane Library、Embase 和 ClinicalTrials.gov 上搜索了相关的随机临床试验 (RCT)。使用具有相应 95% CI 的 Peto OR 计算汇总结果。
结果
分析了 60 项随机对照试验,共纳入 103,034 名患者。含有 ICS 的吸入疗法(Peto OR,0.90;95% CI,0.84-0.97),尤其是三联疗法(Peto OR,0.73;95% CI,0.59-0.91)与全因死亡风险降低相关与不使用 ICS 的吸入疗法相比,COPD 患者。亚组分析显示治疗持续时间 > 6 个月(Peto OR,0.90;95% CI,0.83-0.97)、中剂量 ICSs(Peto OR,0.71;95% CI,0.56-0.91)、低剂量 ICSs(Peto OR,0.88;95% CI,0.79-0.97)和布地奈德(Peto OR,0.75;95% CI,0.59-0.94)参与该关联。这种关联的预测因子包括嗜酸性粒细胞计数≥ 200/μL 或百分比≥ 2%,记录在案的前一年≥ 2 次中度和重度加重病史,2,其中嗜酸性粒细胞计数≥200/μL(Peto OR,0.58;95% CI,0.36-0.95)是最强的预测因子。
解释
包含 ICS 的吸入疗法,尤其是三联疗法,超过 6 个月与 COPD 患者全因死亡风险的降低相关。这种关联的预测因素包括药物因素和患者特征,其中嗜酸性粒细胞计数≥ 200/μL 是最强的预测因素。
试用注册表
普罗斯佩罗;编号:CRD42022304725;网址:https://www.crd.york.ac.uk/prospero/


























 京公网安备 11010802027423号
京公网安备 11010802027423号