当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Highly Enantioselective Iridium-Catalyzed Hydrogenation of o-Amidophenyl Ketones Enabled by 1,2-Diphenylethylenediamine-Derived P,N,N-Ligands with Tertiary Amine Terminus
Organic Letters ( IF 5.2 ) Pub Date : 2022-07-30 , DOI: 10.1021/acs.orglett.2c02316 Yin-Bo Wan 1, 2 , Xiang-Ping Hu 1
Organic Letters ( IF 5.2 ) Pub Date : 2022-07-30 , DOI: 10.1021/acs.orglett.2c02316 Yin-Bo Wan 1, 2 , Xiang-Ping Hu 1
Affiliation
|
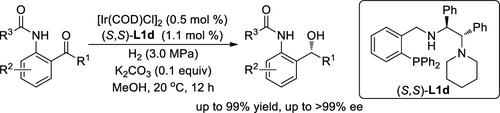
A readily available and highly modular class of chiral P,N,N-ligands based on a structurally flexible nonchiral phosphine-amine framework with an optically active 1,2-diphenylethylenediamine unit bearing a tertiary amine terminus as the chiral source have been developed and successfully applied in the Ir-catalyzed asymmetric hydrogenation of o-amidophenyl ketones. These tridentate P,N,N-ligands exhibited excellent activity, enantioselectivity, and substrate tolerance, thus furnishing various optically active o-amidobenzhydrols in up to 99% yields and with >99% ee. The utility of this protocol has been proven by synthetically diverse product transformation and highly enantioselective production of a rice plant growth regulator, (S)-inabenfide.
中文翻译:

1,2-二苯基乙二胺衍生的具有叔胺末端的 P,N,N-配体实现的高对映选择性铱催化氢化邻氨基苯基酮
基于结构灵活的非手性膦-胺骨架和带有叔胺末端的光学活性 1,2-二苯乙二胺单元作为手性源,一种易于获得且高度模块化的手性 P,N,N-配体已被开发并成功应用于Ir催化的邻氨基苯基酮的不对称氢化。这些三齿 P,N,N-配体表现出优异的活性、对映选择性和底物耐受性,从而以高达 99% 的产率和 >99% 的 ee提供各种光学活性邻氨基苯甲酸。该协议的实用性已通过综合多样化的产品转化和水稻植物生长调节剂 ( S )-inabenfide 的高度对映选择性生产得到证明。
更新日期:2022-07-30
中文翻译:

1,2-二苯基乙二胺衍生的具有叔胺末端的 P,N,N-配体实现的高对映选择性铱催化氢化邻氨基苯基酮
基于结构灵活的非手性膦-胺骨架和带有叔胺末端的光学活性 1,2-二苯乙二胺单元作为手性源,一种易于获得且高度模块化的手性 P,N,N-配体已被开发并成功应用于Ir催化的邻氨基苯基酮的不对称氢化。这些三齿 P,N,N-配体表现出优异的活性、对映选择性和底物耐受性,从而以高达 99% 的产率和 >99% 的 ee提供各种光学活性邻氨基苯甲酸。该协议的实用性已通过综合多样化的产品转化和水稻植物生长调节剂 ( S )-inabenfide 的高度对映选择性生产得到证明。


























 京公网安备 11010802027423号
京公网安备 11010802027423号