当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chemical Conformation of the Essential Glutamate Site of the c-Ring within Thermophilic Bacillus FoF1-ATP Synthase Determined by Solid-State NMR Based on its Isolated c-Ring Structure
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2022-07-29 , DOI: 10.1021/jacs.2c03580 Yasuto Todokoro 1, 2 , Su-Jin Kang 1, 3, 4 , Toshiharu Suzuki 5 , Takahisa Ikegami 6 , Masatsune Kainosho 7 , Masasuke Yoshida 8 , Toshimichi Fujiwara 1 , Hideo Akutsu 1, 3, 6
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2022-07-29 , DOI: 10.1021/jacs.2c03580 Yasuto Todokoro 1, 2 , Su-Jin Kang 1, 3, 4 , Toshiharu Suzuki 5 , Takahisa Ikegami 6 , Masatsune Kainosho 7 , Masasuke Yoshida 8 , Toshimichi Fujiwara 1 , Hideo Akutsu 1, 3, 6
Affiliation
|
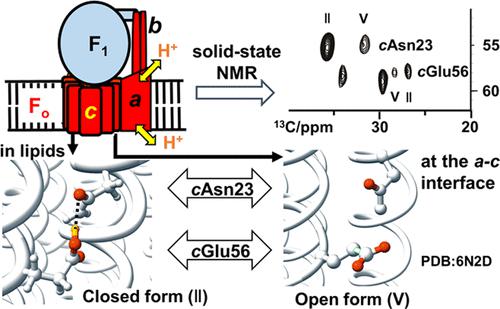
Proton translocation through the membrane-embedded Fo component of F-type ATP synthase (FoF1) is facilitated by the rotation of the Foc-subunit ring (c-ring), carrying protons at essential acidic amino acid residues. Cryo-electron microscopy (Cryo-EM) structures of FoF1 suggest a unique proton translocation mechanism. To elucidate it based on the chemical conformation of the essential acidic residues of the c-ring in FoF1, we determined the structure of the isolated thermophilic Bacillus Fo (tFo) c-ring, consisting of 10 subunits, in membranes by solid-state NMR. This structure contains a distinct proton-locking conformation, wherein Asn23 (cN23) CγO and Glu56 (cE56) CδOH form a hydrogen bond in a closed form. We introduced stereo-array-isotope-labeled (SAIL) Glu and Asn into the tFoc-ring to clarify the chemical conformation of these residues in tFoF1-ATP synthase (tFoF1). Two well-separated 13C signals could be detected for cN23 and cE56 in a 505 kDa membrane protein complex, respectively, thereby suggesting the presence of two distinct chemical conformations. Based on the signal intensity and structure of the tFoc-ring and tFoF1, six pairs of cN23 and cE56 surrounded by membrane lipids take the closed form, whereas the other four in the a–c interface employ the deprotonated open form at a proportion of 87%. This indicates that the a–c interface is highly hydrophilic. The pKa values of the four cE56 residues in the a–c interface were estimated from the cN23 signal intensity in the open and closed forms and distribution of polar residues around each cE56. The results favor a rotation of the c-ring for ATP synthesis.
中文翻译:

基于分离的 c 环结构的固态 NMR 测定嗜热芽孢杆菌 FoF1-ATP 合酶中 c 环必需谷氨酸位点的化学构象
F o c亚基环 ( c环)的旋转促进了质子通过F 型 ATP 合酶 (F o F 1 )的膜包埋 F o组分的易位,在必需酸性氨基酸残基处携带质子。F o F 1的低温电子显微镜 (Cryo-EM) 结构表明了一种独特的质子易位机制。为了阐明它基于F o F 1中c环的必需酸性残基的化学构象,我们确定了分离的嗜热芽孢杆菌F o (tF o) c-环,由 10 个亚基组成,通过固态 NMR 在膜中。该结构包含独特的质子锁定构象,其中 Asn23 ( c N23) C γ O 和 Glu56 ( c E56) C δ OH 以闭合形式形成氢键。我们将立体阵列同位素标记 (SAIL) Glu 和 Asn 引入 tF o c -环以阐明 tF o F 1 -ATP 合酶 (tF o F 1 ) 中这些残基的化学构象。对于c N23 和c,可以检测到两个分离良好的13 C 信号E56 分别位于 505 kDa 的膜蛋白复合物中,从而表明存在两种不同的化学构象。基于 tF o c环和 tF o F 1的信号强度和结构,被膜脂包围的六对c N23 和c E56 采用封闭形式,而a - c界面中的其他四对采用去质子化以 87% 的比例打开表格。这表明a - c界面是高度亲水的。a – c中四个c E56 残基的 p K a值从开放和封闭形式的c N23 信号强度和每个c E56周围的极性残基分布估计界面。结果有利于ATP 合成的c环旋转。
更新日期:2022-07-29
中文翻译:

基于分离的 c 环结构的固态 NMR 测定嗜热芽孢杆菌 FoF1-ATP 合酶中 c 环必需谷氨酸位点的化学构象
F o c亚基环 ( c环)的旋转促进了质子通过F 型 ATP 合酶 (F o F 1 )的膜包埋 F o组分的易位,在必需酸性氨基酸残基处携带质子。F o F 1的低温电子显微镜 (Cryo-EM) 结构表明了一种独特的质子易位机制。为了阐明它基于F o F 1中c环的必需酸性残基的化学构象,我们确定了分离的嗜热芽孢杆菌F o (tF o) c-环,由 10 个亚基组成,通过固态 NMR 在膜中。该结构包含独特的质子锁定构象,其中 Asn23 ( c N23) C γ O 和 Glu56 ( c E56) C δ OH 以闭合形式形成氢键。我们将立体阵列同位素标记 (SAIL) Glu 和 Asn 引入 tF o c -环以阐明 tF o F 1 -ATP 合酶 (tF o F 1 ) 中这些残基的化学构象。对于c N23 和c,可以检测到两个分离良好的13 C 信号E56 分别位于 505 kDa 的膜蛋白复合物中,从而表明存在两种不同的化学构象。基于 tF o c环和 tF o F 1的信号强度和结构,被膜脂包围的六对c N23 和c E56 采用封闭形式,而a - c界面中的其他四对采用去质子化以 87% 的比例打开表格。这表明a - c界面是高度亲水的。a – c中四个c E56 残基的 p K a值从开放和封闭形式的c N23 信号强度和每个c E56周围的极性残基分布估计界面。结果有利于ATP 合成的c环旋转。


























 京公网安备 11010802027423号
京公网安备 11010802027423号