当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetic Solvent Isotope Effect in P450-Mediated Cyclization in Indolactams: Evidence for Branched Reactions and Guide for Their Modulation in Heterocycle Chemoenzymatic Synthesis
ACS Catalysis ( IF 12.9 ) Pub Date : 2022-07-29 , DOI: 10.1021/acscatal.2c02556 Yan Long 1 , Shuo Zheng 1 , Yuxin Feng 1 , Zixuan Yang 1 , Xinlei Xu 1 , Heng Song 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2022-07-29 , DOI: 10.1021/acscatal.2c02556 Yan Long 1 , Shuo Zheng 1 , Yuxin Feng 1 , Zixuan Yang 1 , Xinlei Xu 1 , Heng Song 1
Affiliation
|
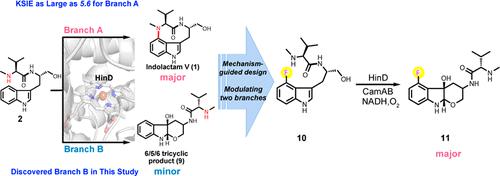
Cytochrome P450s demonstrate potential as biocatalysts for selective C–H bond functionalization in heterocycle synthesis. P450 monooxygenase (HinD) can catalyze cyclization for the biosynthesis of indolactam-like natural products. Moreover, the modification of N13 in the substrates leads to the formation of 6/5/6 tricyclic products as the minor product, which demonstrates potential for cancer treatment. However, the biosynthesis of indole-fused 6/5/6 tricyclic products is challenging as relatively few studies focus on the modulation of the minor products from P450 reactions to become dominant products for their bioproduction. Here, the isotopically sensitive branching method was employed to identify the conversion to 6/5/6 tricyclic products as the minor branch in wild-type HinD catalysis, originating from a common intermediate, in addition to the formation of 9-membered indolactam. The observed large kinetic solvent isotope effect (∼5.6) revealed that catalytic flow between two branches is sensitive and that the original minor product can be inverted to the dominant product by directing catalytic flow from the original major branch to the minor branch. Finally, 6/5/6 tricyclic products can be modulated and chemoenzymatically prepared as the major product. Data offer an insight into the rational design for the chemo-enzymatic synthesis of heterocyclic compounds.
中文翻译:

P450介导的吲哚内酰胺环化中的动力学溶剂同位素效应:支链反应的证据及其在杂环化学酶合成中的调节指南
细胞色素 P450 在杂环合成中表现出作为选择性 C-H 键功能化的生物催化剂的潜力。P450 单加氧酶 (HinD) 可催化环化反应,用于生物合成吲哚内酰胺类天然产物。此外,底物中N13的修饰导致形成6/5/6三环产物作为次要产物,这证明了癌症治疗的潜力。然而,吲哚稠合的 6/5/6 三环产物的生物合成具有挑战性,因为相对较少的研究关注调节 P450 反应中的次要产物成为其生物生产的主要产物。在这里,同位素敏感的支化方法被用来识别转化为 6/5/6 三环产物作为野生型 HinD 催化中的次要分支,源自一个常见的中间体,除了形成 9 元吲哚内酰胺。观察到的大动力学溶剂同位素效应(~5.6)表明两个分支之间的催化流动是敏感的,并且可以通过将催化流从原始主要分支引导到次要分支来将原始次要产物转化为主要产物。最后,6/5/6三环产物可以作为主要产物进行调制和化学酶法制备。数据提供了对杂环化合物化学酶促合成的合理设计的深入了解。最后,6/5/6三环产物可以作为主要产物进行调制和化学酶法制备。数据提供了对杂环化合物化学酶促合成的合理设计的深入了解。最后,6/5/6三环产物可以作为主要产物进行调制和化学酶法制备。数据提供了对杂环化合物化学酶促合成的合理设计的深入了解。
更新日期:2022-07-29
中文翻译:

P450介导的吲哚内酰胺环化中的动力学溶剂同位素效应:支链反应的证据及其在杂环化学酶合成中的调节指南
细胞色素 P450 在杂环合成中表现出作为选择性 C-H 键功能化的生物催化剂的潜力。P450 单加氧酶 (HinD) 可催化环化反应,用于生物合成吲哚内酰胺类天然产物。此外,底物中N13的修饰导致形成6/5/6三环产物作为次要产物,这证明了癌症治疗的潜力。然而,吲哚稠合的 6/5/6 三环产物的生物合成具有挑战性,因为相对较少的研究关注调节 P450 反应中的次要产物成为其生物生产的主要产物。在这里,同位素敏感的支化方法被用来识别转化为 6/5/6 三环产物作为野生型 HinD 催化中的次要分支,源自一个常见的中间体,除了形成 9 元吲哚内酰胺。观察到的大动力学溶剂同位素效应(~5.6)表明两个分支之间的催化流动是敏感的,并且可以通过将催化流从原始主要分支引导到次要分支来将原始次要产物转化为主要产物。最后,6/5/6三环产物可以作为主要产物进行调制和化学酶法制备。数据提供了对杂环化合物化学酶促合成的合理设计的深入了解。最后,6/5/6三环产物可以作为主要产物进行调制和化学酶法制备。数据提供了对杂环化合物化学酶促合成的合理设计的深入了解。最后,6/5/6三环产物可以作为主要产物进行调制和化学酶法制备。数据提供了对杂环化合物化学酶促合成的合理设计的深入了解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号