当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Elucidating Cooperative Interactions between Grafted Amines and Tin or Titanium Sites on Silica
ACS Catalysis ( IF 12.9 ) Pub Date : 2022-07-28 , DOI: 10.1021/acscatal.2c02276 Christine Khoury 1 , Samuel Holton 2 , Dina Shpasser 1 , Elior Hallo 1 , Ambarish Kulkarni 2 , Friederike C. Jentoft 3 , Oz M. Gazit 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2022-07-28 , DOI: 10.1021/acscatal.2c02276 Christine Khoury 1 , Samuel Holton 2 , Dina Shpasser 1 , Elior Hallo 1 , Ambarish Kulkarni 2 , Friederike C. Jentoft 3 , Oz M. Gazit 1
Affiliation
|
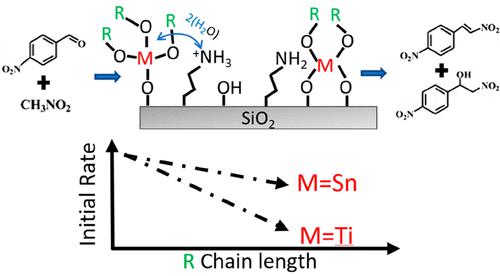
The efficient promotion of cooperative catalytic interactions on solid surfaces can be of great benefit for a range of important reactions. Herein, we demonstrate that the cooperative interactions of isolated tin (Sn) and titanium (Ti) sites on silica with grafted primary amines (NH2) can be tuned by changing the immediate chemical environment of the metal sites (M). We show that, by tethering various size organic ligands (RO) to the M sites, we can govern the interactions between the sites as measured by the presence of NH3+. We show that the concentration of NH3+ is directly correlated with the activity of the model Henry reaction. We further find that the selectivity to the olefinic product increased from 59% for the cooperative interactions of grafted NH2 and surface silanols to 84–92% for the cooperative interactions between grafted NH2 and the isolated Sn or Ti sites. An analysis by DFT shows that these cooperative interactions are enabled by the presence of a trace amount (two molecules per M site) of water near the metal sites and a resulting hydrolysis, which depends on the hydrophobicity of the RO group and the nature of the metal. Hence, the current work provides advanced molecular-level insights into the underlying principles of cooperative interactions on a solid surface and guidance for governing such interactions by tuning the chemical environment.
中文翻译:

阐明接枝胺与二氧化硅上的锡或钛位点之间的协同作用
有效促进固体表面上的协同催化相互作用对一系列重要反应非常有益。在此,我们证明了二氧化硅上孤立的锡 (Sn) 和钛 (Ti) 位点与接枝伯胺 (NH 2 ) 的协同相互作用可以通过改变金属位点 (M) 的直接化学环境来调节。我们表明,通过将各种大小的有机配体 (RO) 束缚到 M 位点,我们可以控制位点之间的相互作用,如通过 NH 3 +的存在所测量的那样。我们证明了 NH 3 +与模型亨利反应的活性直接相关。我们进一步发现,烯烃产物的选择性从接枝 NH 2和表面硅烷醇的协同作用的 59% 提高到接枝 NH 2之间的协同作用的 84-92%和孤立的 Sn 或 Ti 位点。DFT 分析表明,这些协同相互作用是通过金属位点附近存在微量水(每个 M 位点两个分子)以及由此产生的水解来实现的,这取决于 RO 基团的疏水性和 RO 基团的性质。金属。因此,目前的工作为固体表面上协同相互作用的基本原理提供了先进的分子水平见解,并为通过调整化学环境来控制这种相互作用提供了指导。
更新日期:2022-07-28
中文翻译:

阐明接枝胺与二氧化硅上的锡或钛位点之间的协同作用
有效促进固体表面上的协同催化相互作用对一系列重要反应非常有益。在此,我们证明了二氧化硅上孤立的锡 (Sn) 和钛 (Ti) 位点与接枝伯胺 (NH 2 ) 的协同相互作用可以通过改变金属位点 (M) 的直接化学环境来调节。我们表明,通过将各种大小的有机配体 (RO) 束缚到 M 位点,我们可以控制位点之间的相互作用,如通过 NH 3 +的存在所测量的那样。我们证明了 NH 3 +与模型亨利反应的活性直接相关。我们进一步发现,烯烃产物的选择性从接枝 NH 2和表面硅烷醇的协同作用的 59% 提高到接枝 NH 2之间的协同作用的 84-92%和孤立的 Sn 或 Ti 位点。DFT 分析表明,这些协同相互作用是通过金属位点附近存在微量水(每个 M 位点两个分子)以及由此产生的水解来实现的,这取决于 RO 基团的疏水性和 RO 基团的性质。金属。因此,目前的工作为固体表面上协同相互作用的基本原理提供了先进的分子水平见解,并为通过调整化学环境来控制这种相互作用提供了指导。


























 京公网安备 11010802027423号
京公网安备 11010802027423号