当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Determination and Analysis of Solubility of 2-Bromo-9-fluorenone in 10 Different Organic Solvents and Three Binary Solvent Mixtures at Different Temperatures (T = 278.15–323.15 K)
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2022-07-27 , DOI: 10.1021/acs.jced.2c00163 Yanjuan Peng 1 , Ziteng Wang 1 , Wenhao Jiao 1 , Xinxin Cheng 1 , Jingjing Yang 1 , Yonghong Hu 1 , Li Mi 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2022-07-27 , DOI: 10.1021/acs.jced.2c00163 Yanjuan Peng 1 , Ziteng Wang 1 , Wenhao Jiao 1 , Xinxin Cheng 1 , Jingjing Yang 1 , Yonghong Hu 1 , Li Mi 1
Affiliation
|
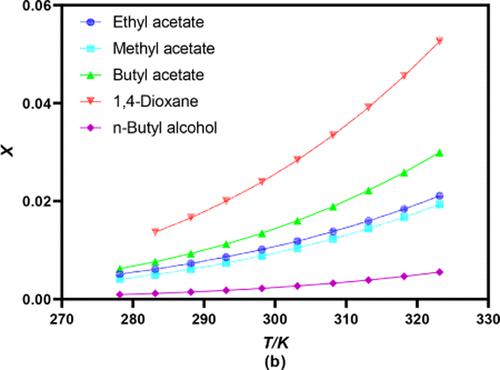
This article deals with the saturated solubility and solvation behavior of 2-bromo-9-fluorenone in 10 industrial common solvents and three binary mixed solvents by means of experimental and mathematical correlations. Solubility experiments were investigated using the static equilibrium method combined with high-performance liquid chromatography (HPLC) from 278.15 to 323.15 K at atmospheric pressure. The results showed that the maximum and minimum solubility of 2-bromo-9-fluorenone was 0.0526 (mole fraction) in 1,4-dioxane at 323.15 K and 0.0002 (mole fraction) in methanol at 278.15 K, respectively, and the solubility increased with the increase in temperature in all solvents. Furthermore, the solubility data of 2-bromo-9-fluorenone was analyzed using the Apelblat model, the Buchowski–Ksiazaczak λh model in pure solvents, CNIBS/R-K model, and the Jouyban–Acree model in binary solvent mixtures. By error comparison, the Apelblat model and the CNIBS/R-K model can better predict the solubility of 2-bromo-9-fluorenone. In addition, the solvation behavior was illustrated by the KAT-LSER model in pure solvents, and the result shows that the cohesive energy density in the solution is the main factor affecting 2-bromo-9-fluorenone. Therefore, the solubility data is very important for optimizing the extraction process and recrystallizing 2-bromo-9-fluorenone to obtain a higher yield in industry production.
中文翻译:

2-Bromo-9-芴酮在不同温度下(T = 278.15–323.15 K)在 10 种不同有机溶剂和三种二元溶剂混合物中溶解度的测定和分析
本文通过实验和数学相关性研究了2-溴-9-芴酮在10种工业常用溶剂和三种二元混合溶剂中的饱和溶解度和溶剂化行为。使用静态平衡法结合高效液相色谱 (HPLC) 在大气压下从 278.15 到 323.15 K 研究溶解度实验。结果表明,2-溴-9-芴酮在323.15 K时在1,4-二恶烷中的最大和最小溶解度分别为0.0526(摩尔分数)和在278.15 K时在甲醇中的0.0002(摩尔分数),溶解度增加随着所有溶剂中温度的升高。此外,使用 Apelblat 模型 Buchowski-Ksiazaczak λ h分析了 2-溴-9-芴酮的溶解度数据纯溶剂模型、CNIBS/RK 模型和二元溶剂混合物中的 Jouyban-Acree 模型。通过误差比较,Apelblat模型和CNIBS/RK模型可以更好地预测2-溴-9-芴酮的溶解度。此外,通过KAT-LSER模型说明了纯溶剂中的溶剂化行为,结果表明溶液中的内聚能密度是影响2-溴-9-芴酮的主要因素。因此,溶解度数据对于优化提取工艺和重结晶2-溴-9-芴酮以获得更高的工业生产收率非常重要。
更新日期:2022-07-27
中文翻译:

2-Bromo-9-芴酮在不同温度下(T = 278.15–323.15 K)在 10 种不同有机溶剂和三种二元溶剂混合物中溶解度的测定和分析
本文通过实验和数学相关性研究了2-溴-9-芴酮在10种工业常用溶剂和三种二元混合溶剂中的饱和溶解度和溶剂化行为。使用静态平衡法结合高效液相色谱 (HPLC) 在大气压下从 278.15 到 323.15 K 研究溶解度实验。结果表明,2-溴-9-芴酮在323.15 K时在1,4-二恶烷中的最大和最小溶解度分别为0.0526(摩尔分数)和在278.15 K时在甲醇中的0.0002(摩尔分数),溶解度增加随着所有溶剂中温度的升高。此外,使用 Apelblat 模型 Buchowski-Ksiazaczak λ h分析了 2-溴-9-芴酮的溶解度数据纯溶剂模型、CNIBS/RK 模型和二元溶剂混合物中的 Jouyban-Acree 模型。通过误差比较,Apelblat模型和CNIBS/RK模型可以更好地预测2-溴-9-芴酮的溶解度。此外,通过KAT-LSER模型说明了纯溶剂中的溶剂化行为,结果表明溶液中的内聚能密度是影响2-溴-9-芴酮的主要因素。因此,溶解度数据对于优化提取工艺和重结晶2-溴-9-芴酮以获得更高的工业生产收率非常重要。


























 京公网安备 11010802027423号
京公网安备 11010802027423号